推薦產品
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.509 (lit.)
bp
111-112 °C/18 mmHg (lit.)
密度
1.078 g/mL at 25 °C (lit.)
官能基
ketone
SMILES 字串
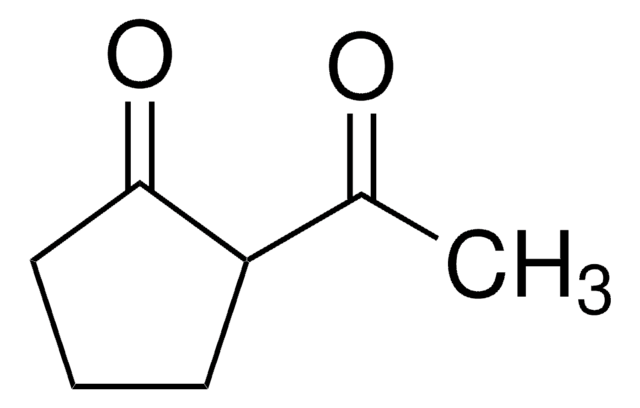
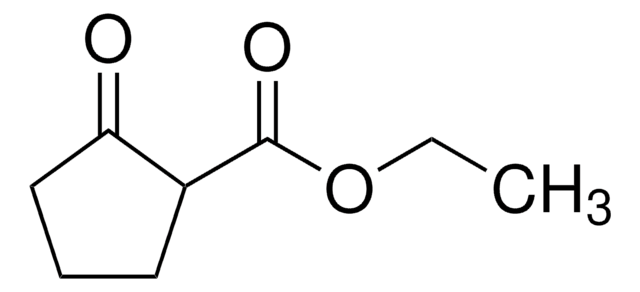
CC(=O)C1CCCCC1=O
InChI
1S/C8H12O2/c1-6(9)7-4-2-3-5-8(7)10/h7H,2-5H2,1H3
InChI 密鑰
OEKATORRSPXJHE-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
已经研究了2-乙酰基环己酮(ACHE)在水中的酮-烯醇互变异构现象。
應用
2-乙酰环己酮被用于合成苯氨基乙醇胺。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
174.2 °F - closed cup
閃點(°C)
79 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
客戶也查看了
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2680-2688 (2003-03-29)
The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) was studied in water under different experimental conditions. By contrast with other previously studied beta-diketones, the keto-enol interconversion in the ACHE system is a slow process. Under equilibrium conditions, the analysis of the absorbance
Cédric Bouteiller et al.
Organic & biomolecular chemistry, 8(5), 1111-1120 (2010-02-19)
An operationally simple and concise synthesis of anilinoethanolamines, as NMDA NR2B receptor antagonist ifenprodil analogues, was developed via a copper-catalyzed amination of the corresponding bromoarene. Coupling was achieved with linear primary alkylamines, alpha,omega-diamines, hexanolamine and benzophenone imine, as well as
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2689-2697 (2003-03-29)
The kinetic study of the nitrosation of the enol of 2-acetylcyclohexanone (ACHE) has been performed in aqueous acid media in the absence and presence of alpha- and beta-cyclodextrin. The reaction is first-order with respect to both reactants concentration: [nitrite] and
An alternative to the classical α-arylation: the transfer of an intact 2-iodoaryl from ArI(O₂CCF₃)₂.
Zhiyu Jia et al.
Angewandte Chemie (International ed. in English), 53(42), 11298-11301 (2014-09-10)
The α-arylation of carbonyl compounds is generally accomplished under basic conditions, both under metal catalysis and via aryl transfer from the diaryl λ(3)-iodanes. Here, we describe an alternative metal-free α-arylation using ArI(O2CCF3)2 as the source of a 2-iodoaryl group. The
Masaharu Fujita et al.
Journal of applied toxicology : JAT, 39(2), 191-208 (2018-09-18)
The amino acid derivative reactivity assay (ADRA) is an in chemico alternative to animal testing for skin sensitization that solves certain problems found in the use of the direct peptide reactivity assay (DPRA). During a recent validation study conducted at
文章
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務

![[4,4′-双(1,1-二甲基乙基)-2,2′-联吡啶]二氯化镍(II)](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)