推薦產品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.536 (lit.)
bp
193 °C (lit.)
密度
1.164 g/mL at 25 °C (lit.)
官能基
chloro
SMILES 字串
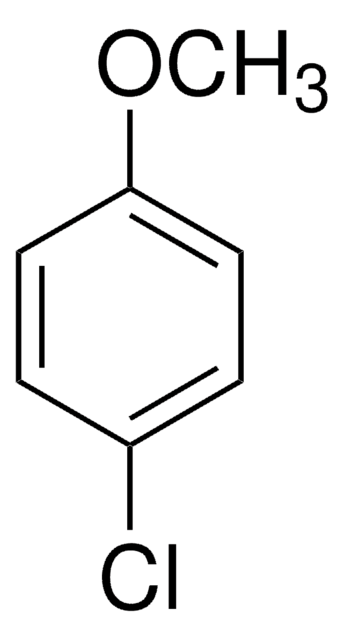
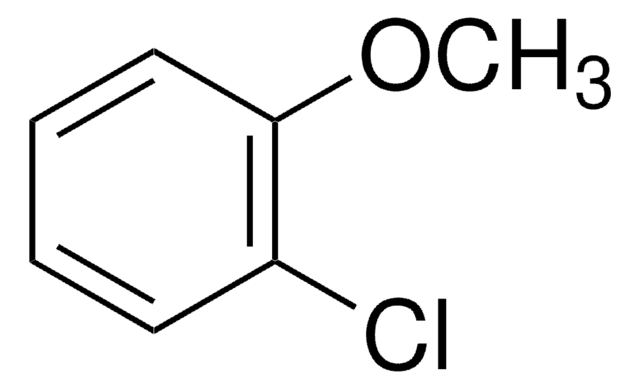
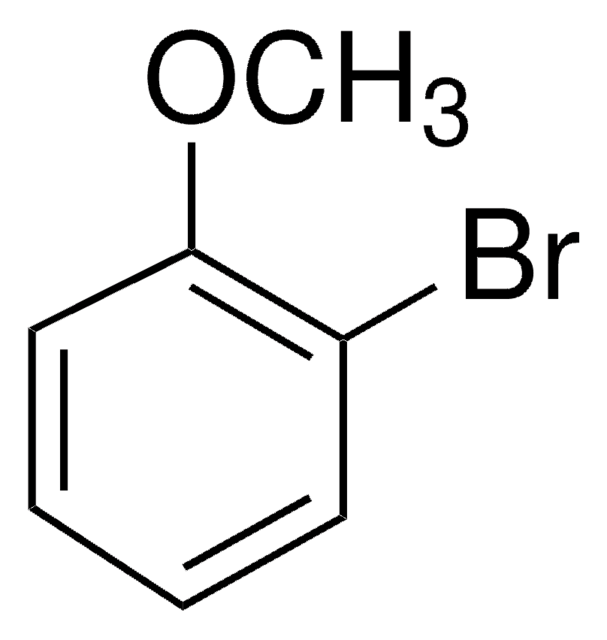
COc1cccc(Cl)c1
InChI
1S/C7H7ClO/c1-9-7-4-2-3-6(8)5-7/h2-5H,1H3
InChI 密鑰
YUKILTJWFRTXGB-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
One-step preparation of 3-chloroanisole from the corresponding 3-substituted nitrobenzene has been reported.
應用
3-Chloroanisole was employed as starting reagent in the regioselective synthesis of 4- and 7-alkoxyindoles. It was also employed as electrolyte additive for the overcharging protection of Li-ion cell.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
163.4 °F - closed cup
閃點(°C)
73 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
3-Chloroanisole for overcharge protection of a Li-ion cell.
Lee Y-G and Cho J.
Electrochimica Acta, 52(25), 7404-7408 (2007)
Roberto Sanz et al.
The Journal of organic chemistry, 72(14), 5113-5118 (2007-06-15)
An efficient and regioselective synthesis of 4- and 7-alkoxyindoles has been developed from commercially available starting materials such as 3-halophenols and 3-chloroanisole. Directed ortho-metalation followed by two palladium-catalyzed processes, a Sonogashira coupling and a tandem amination/cyclization reaction, allows the synthesis
One-step preparation of some 3-substituted anisoles.
Zilberman J.
Organic Process Research & Development, 7(3), 303-305 (2003)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務