推薦產品
化驗
99%
形狀
crystals
mp
60-64 °C (lit.)
官能基
ketone
thioether
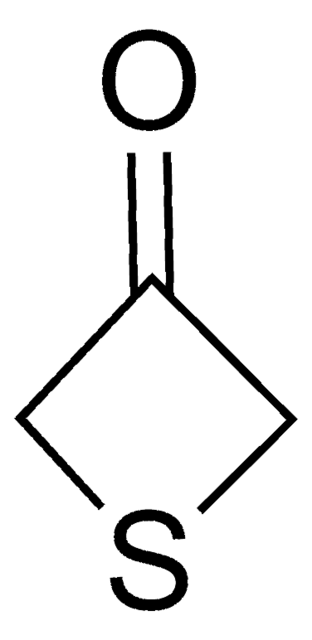
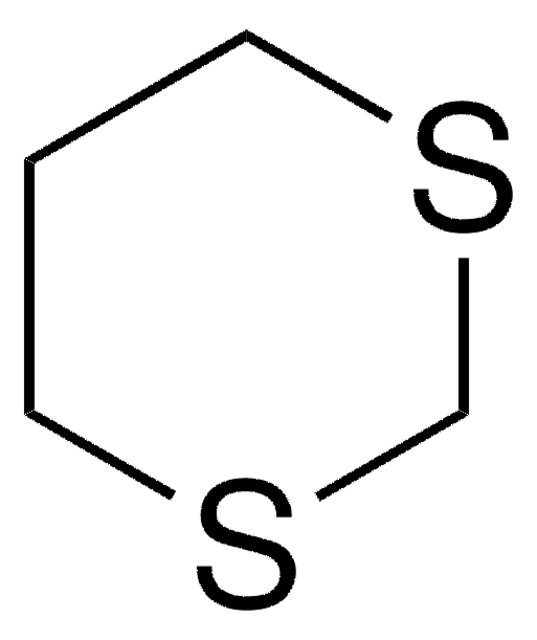
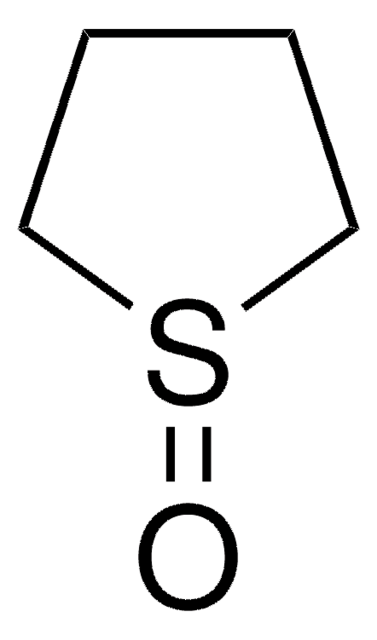
SMILES 字串
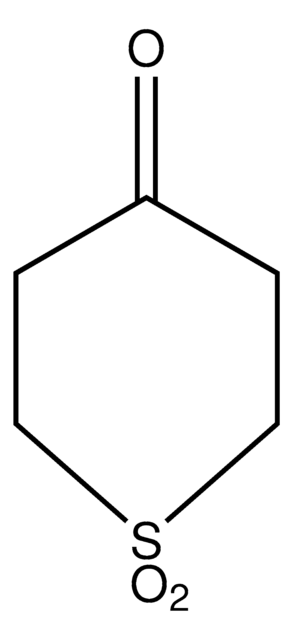
O=C1CCSCC1
InChI
1S/C5H8OS/c6-5-1-3-7-4-2-5/h1-4H2
InChI 密鑰
OVRJVKCZJCNSOW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
The diastereoselectivity of the aldol reaction of tetrahydro-4H-thiopyran-4-one has been studied.
應用
用于各种缩合反应,制备二肽、螺咪唑啉酮、四氢咔唑和 α-羟基酯。
Tetrahydro-4H-thiopyran-4-one was used in the preparation of meso 1,9-diketones.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Dale E Ward et al.
The Journal of organic chemistry, 67(5), 1618-1629 (2002-03-02)
The diastereoselectivity of the aldol reaction of tetrahydro-4H-thiopyran-4-one (3) with 1,4-dioxa-8-thiaspiro[4.5]decane-6-carboxaldehyde (9a) under a variety of conditions is examined. Under optimized conditions, three of the four possible diastereomers from this aldol reaction can be obtained selectively (3-16:1). Reactions of 9a
Dale E Ward et al.
Organic letters, 8(12), 2631-2634 (2006-06-02)
Meso 1,9-diketones (six to seven stereocenters) are readily obtained by stepwise or simultaneous two-directional aldol reactions of tetrahydro-4H-thiopyran-4-one with a thiopyran-derived aldehyde or dialdehyde. Enantioselective enolizations of these diketones with the lithium amide from (R,R)-bis(1-phenylethyl)amine occur with simultaneous kinetic resolution
Synlett, 1605-1605 (2007)
Synthesis, 672-672 (1994)
Journal of Heterocyclic Chemistry, 30, 81-81 (1993)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務