推薦產品
蒸汽密度
2.1 (vs air)
蒸汽壓力
0.2 mmHg ( 20 °C)
化驗
≥99%
形狀
liquid
自燃溫度
1436 °F
expl. lim.
17 %
雜質
≤0.5% water (Karl Fischer)
燃燒殘留物
≤0.05% (as SO4)
折射率
n20/D 1.454 (lit.)
bp
170 °C (lit.)
69-70 °C/10 mmHg
mp
10-11 °C (lit.)
密度
1.012 g/mL at 25 °C (lit.)
正離子痕跡
Fe: ≤10 mg/kg
官能基
amine
hydroxyl
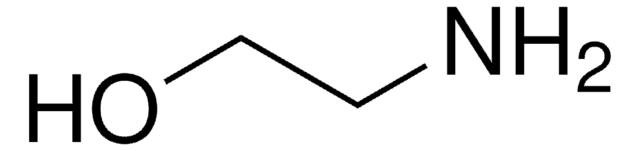
SMILES 字串
NCCO
InChI
1S/C2H7NO/c3-1-2-4/h4H,1-3H2
InChI 密鑰
HZAXFHJVJLSVMW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
- 氯铝酸盐离子液体的催化性能和酸分析:探索油合成中乙醇胺对离子液体催化活性的影响(Hu et al., 2024)。
其他說明
货号 15014-4X2.5L的产品即将停产。请订购15014-2.5L单瓶装,因为其具体参数与上述产品本质相同。
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
195.8 °F - Pensky-Martens closed cup
閃點(°C)
91 °C - Pensky-Martens closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
J E Vance
Biochimica et biophysica acta, 1045(2), 128-134 (1990-07-16)
Monolayer cultures of rat hepatocytes have been examined for their ability to secrete ethanolamine plasmalogen as a component of nascent lipoproteins. In culture medium from these cells, ethanolamine plasmalogen comprises approx. 20-30% of total ethanolamine glycerophospholipids when measured either as
H Perschak et al.
Human neurobiology, 6(3), 191-194 (1987-01-01)
Concentrations of putative neuroactive substances glutamate, aspartate, gamma-aminobutyric acid, glycine, proline and ethanolamine were determined in ventricular cerebrospinal fluid collected in patients suffering from Parkinson's disease, pain syndromes or cerebellar tremor. Values are similar to those given in the literature
Gabriel da Silva
The journal of physical chemistry. A, 116(45), 10980-10986 (2012-09-22)
The alkanolamine 2-aminoethanol (NH(2)CH(2)CH(2)OH), otherwise known as monoethanolamine (MEA), is a widely used solvent for carbon capture, yet relatively little is known about its atmospheric chemistry. The hydroxyl radical initiated oxidation of MEA is thought to predominantly form the α-aminoalkyl
Danielle A Garsin
Nature reviews. Microbiology, 8(4), 290-295 (2010-03-18)
Ethanolamine is a compound that can be readily derived from cell membranes and that some bacteria can use as a source of carbon and/or nitrogen. The complex biology and chemistry of this process has been under investigation since the 1970s
Ye-Jin Hwang et al.
Advanced materials (Deerfield Beach, Fla.), 27(31), 4578-4584 (2015-07-03)
By controlling the polymer/polymer blend self-organization rate, all-polymer solar cells composed of a high-mobility, crystalline, naphthalene diimide-selenophene copolymer acceptor and a benzodithiophene-thieno[3,4-b]thiophene copolymer donor are achieved with a record 7.7% power conversion efficiency and a record short-circuit current density (18.8
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務