推薦產品
品質等級
化驗
98%
形狀
solid
mp
141-143 °C (lit.)
溶解度
ethanol: soluble 10 mg/mL, clear, light yellow to yellow
官能基
ketone
SMILES 字串
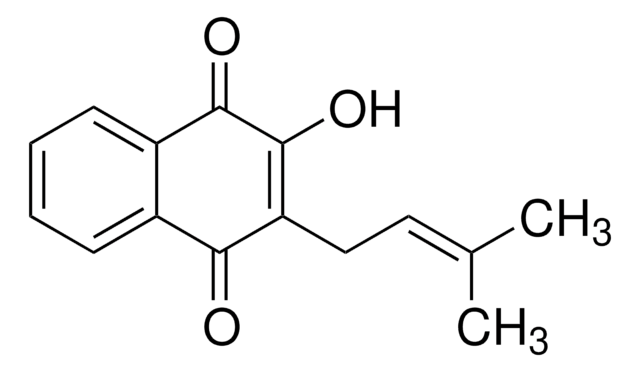
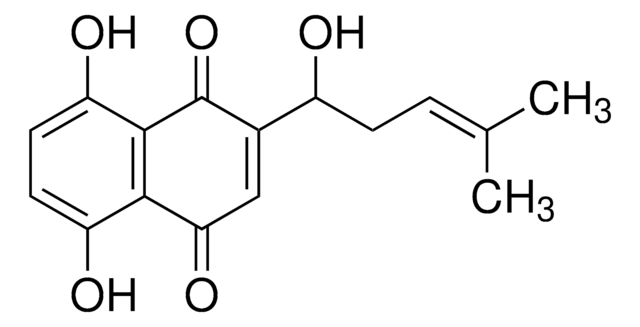
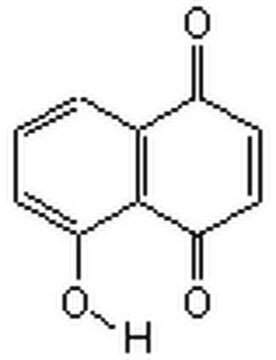
C\C(C)=C\CC1=C(O)C(=O)c2ccccc2C1=O
InChI
1S/C15H14O3/c1-9(2)7-8-12-13(16)10-5-3-4-6-11(10)14(17)15(12)18/h3-7,18H,8H2,1-2H3
InChI 密鑰
CIEYTVIYYGTCCI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
拉帕醇是一种天然萘醌化合物,来源于紫葳科(黄钟木属)。
應用
拉帕醇用于合成拉帕醇金属配合物 。
生化/生理作用
拉帕醇对许多病原体具有抗菌属性 。具有抗炎、镇痛及抗菌属性 。它是黑腹果蝇杂合子 上皮性肿瘤的抑制剂。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
客戶也查看了
Ricardo Vessecchi et al.
Journal of mass spectrometry : JMS, 47(12), 1648-1659 (2013-01-03)
In order to understand the influence of alkyl side chains on the gas-phase reactivity of 1,4-naphthoquinone derivatives, some 2-hydroxy-1,4-naphthoquinone derivatives have been prepared and studied by electrospray ionization tandem mass spectrometry in combination with computational quantum chemistry calculations. Protonation and
Cristian Salas et al.
Bioorganic & medicinal chemistry, 16(2), 668-674 (2007-11-22)
Derivatives of natural quinones with biological activities, such as lapachol, alpha- and beta-lapachones, have been synthesized and their trypanocidal activity evaluated in vitro in Trypanosoma cruzi cells. All tested compounds inhibited epimastigote growth and trypomastigote viability. Several compounds showed similar
Eduardo J S Salustiano et al.
Investigational new drugs, 28(2), 139-144 (2009-03-04)
The pentacyclic 1,4-naphthoquinones 1a-d were cytotoxic (IC(50) approximately 2-7 microM) to human leukemic cell lines K562 (oxidative stress-resistant), Lucena-1 (MDR phenotype) and Daudi. Fresh leukemic cells obtained from patients, some with the MDR phenotype, were also sensitive to these compounds.
Lu Bai et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 944, 128-135 (2013-12-10)
Lapachol is a natural naphthoquinone compound derived from Bignoniaceae (Tabebuia sp.) that possesses a range of significant biological activities. Nine phase I and four phase II metabolites of lapachol in rat bile were firstly elucidated and identified using a sensitive
Renato A S Oliveira et al.
International immunopharmacology, 10(11), 1463-1473 (2010-09-15)
The present study reports the anti-mycobacterial activity of 2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthoquinone (lapachol) as well as its influence on macrophage functions. Lapachol (L) did not induce apoptosis/necrosis of THP-1 macrophages at ≤32 μg/mL. Mycobacterium avium liquid growth was arrested by ≥32 μg/mL and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務