全部照片(1)
About This Item
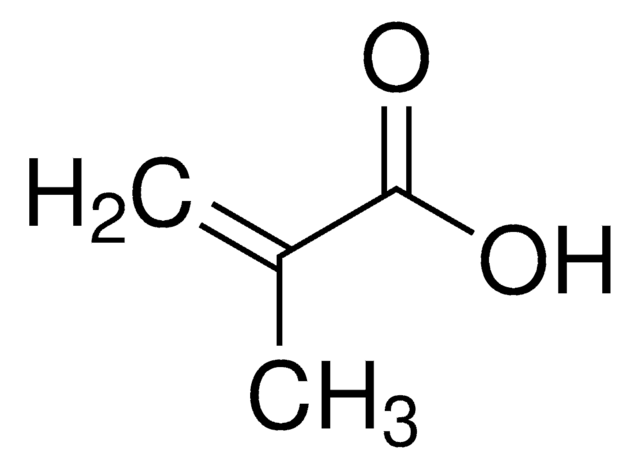
線性公式:
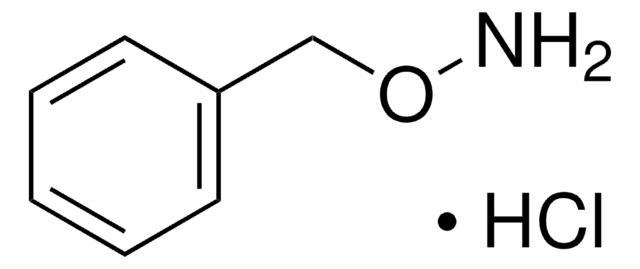
C6H5CH2NHOH · HCl
CAS號碼:
分子量::
159.61
Beilstein:
507948
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
等級
puriss.
品質等級
化驗
≥99.0% (AT)
形狀
solid
mp
~105 °C
108-110 °C (lit.)
官能基
amine
phenyl
SMILES 字串
Cl.ONCc1ccccc1
InChI
1S/C7H9NO.ClH/c9-8-6-7-4-2-1-3-5-7;/h1-5,8-9H,6H2;1H
InChI 密鑰
YSNXOQGDHGUKCZ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
N-Benzylhydroxylamine hydrochloride was used in the synthesis of sugar derived nitrones. It was used as starting reagent in the synthesis of fluoro isoxazoline and isoxazolidine derivatives using flouro nitrone.
生化/生理作用
N-Benzylhydroxylamine is a potential pharmacological agent in the prevention and progression of acrolein-induced damage to the retinal pigment epithelium.
其他說明
从羰基化合物合成硝酮以及与烯烃的 [2+3]-环加成生成异噁唑烷(通用中间体);烯丙基酯的钯催化羟胺化反应。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
R Herrera et al.
The Journal of organic chemistry, 66(4), 1252-1263 (2001-04-21)
Captodative olefins 1-acetylvinyl carboxylates proved to be highly regioselective dipolarophiles in 1,3-dipolar cycloadditon to propionitrile oxide, arylphenylnitrile imines, diazoalkanes, and nitrones to yield the corresponding 5-substituted heterocycles. The addition of the latter was also stereoselective, being slightly susceptible to steric
B.J. Wakefield
Sci. Synth., 11, 229-229 (2002)
Ionic liquid mediated synthesis of some novel fluoro isoxazolidine and isoxazoline derivatives using N-benzyl fluoro nitrone via cycloaddition reaction and their antimicrobial activities.
Chakraborty B, et al.
Indian J. Chem. B, 52(10), 1342-1351 (2013)
T. Kawakami et al.
Bulletin of the Chemical Society of Japan, 73, 2423-2423 (2000)
J.E. Baldwin et al.
Tetrahedron, 42, 3097-3097 (1986)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務