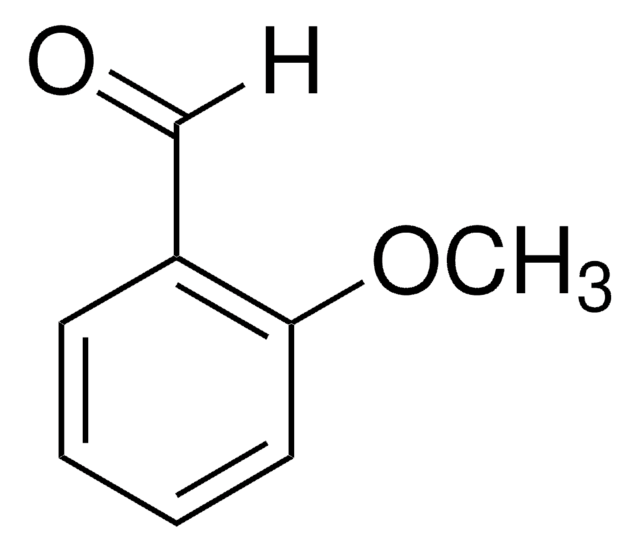
推薦產品
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.553 (lit.)
bp
143 °C/50 mmHg (lit.)
密度
1.117 g/mL at 20 °C (lit.)
官能基
aldehyde
SMILES 字串
[H]C(=O)c1cccc(OC)c1
InChI
1S/C8H8O2/c1-10-8-4-2-3-7(5-8)6-9/h2-6H,1H3
InChI 密鑰
WMPDAIZRQDCGFH-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
间茴香醛对假丝酵母菌(包耐吡咯菌株)有良好的抗真菌活性。.
间茴香醛属于苯甲醛类,可用作强效抗真菌剂和复杂芳香化合物的起始原料。
间茴香醛属于苯甲醛类,可用作强效抗真菌剂和复杂芳香化合物的起始原料。
應用
在正相硅胶色谱中,m-茴香醛可用作香兰素单-13C同位素的洗脱液。它还被用作4-(甲基亚硝基氨基)-1-(3-吡啶基)-1-丁酮(NNK)代谢的抑制剂。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Inhibition of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by dietary benzaldehydes.
M A Morse et al.
Cancer letters, 97(2), 255-261 (1995-11-06)
As part of a routine screening assay, benzaldehyde was found to inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism. Consequently, the effects of benzaldehyde and several structurally related compounds on NNK metabolism were examined in murine hepatic and pulmonary microsomes. All test compounds inhibited
Sheikh Shreaz et al.
Microbial pathogenesis, 51(4), 277-284 (2011-06-15)
Attention has been drawn to evaluate the antifungal activity of p-anisaldehyde (1), o-anisaldehyde (2) and m-anisaldehyde (3). To put forward this approach, antifungal activity has been assessed in thirty six fluconazole-sensitive and eleven fluconazole-resistant Candida isolates. Growth and sensitivity of
Eliot P Botosoa et al.
Journal of chromatography. A, 1216(42), 7043-7048 (2009-09-15)
Quantitative isotopic (13)C NMR at natural abundance has been used to determine the site-by-site (13)C/(12)C ratios in vanillin and a number of related compounds eluted from silica gel chromatography columns under similar conditions. Head-to-tail isotope fractionation is observed in all
Chao-Bin Xue et al.
Bioorganic & medicinal chemistry, 15(5), 2006-2015 (2007-01-30)
Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-quinones. Inhibition of PO may provide a basis for novel environmentally friendly
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務