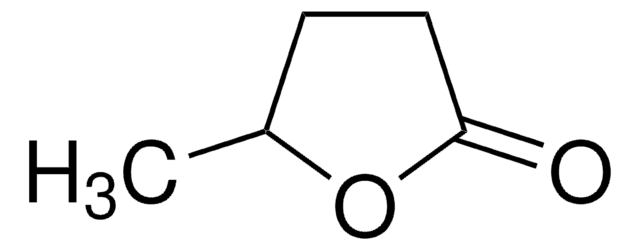
推薦產品
品質等級
化驗
98%
折射率
n20/D 1.432 (lit.)
bp
78-81 °C/10 mmHg (lit.)
溶解度
THF: soluble
官能基
ester
SMILES 字串
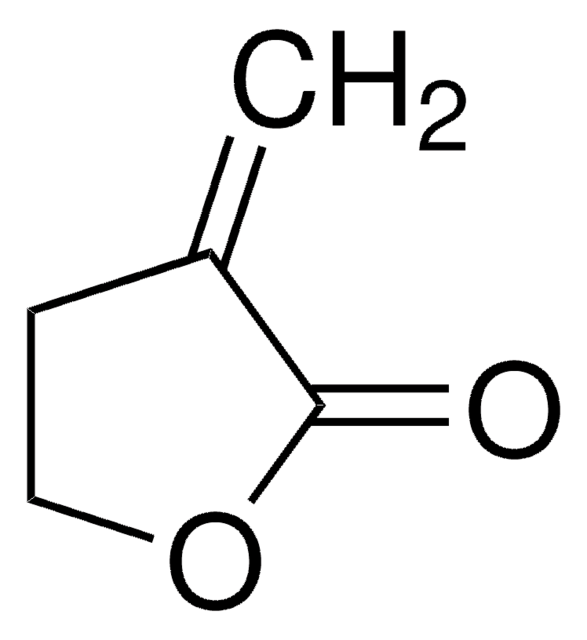
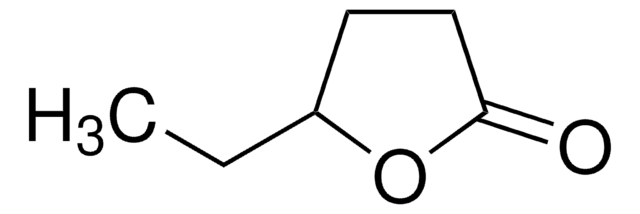
CC1CCOC1=O
InChI
1S/C5H8O2/c1-4-2-3-7-5(4)6/h4H,2-3H2,1H3
InChI 密鑰
QGLBZNZGBLRJGS-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
α-甲基-γ-丁内酯发生苄基化反应生成外消旋α-苄基-α-甲基-γ-丁内酯。
應用
以α-甲基-γ-丁内酯为模型化合物,在交叉实验中研究了毛果芸香碱的热力学优势反应位点。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
163.4 °F - closed cup
閃點(°C)
73 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
M Satterfield et al.
Journal of the American Society for Mass Spectrometry, 10(3), 209-216 (1999-03-09)
Analysis of the sites of reaction of a biologically important compound, pilocarpine, a molecule with imidazole and butyrolactone rings connected by a methylene bridge, has been accomplished in a quadrupole ion trap with the aim of characterizing its structure/reactivity relationships.
Eric B Gonzales et al.
The Journal of pharmacology and experimental therapeutics, 309(2), 677-683 (2004-01-27)
Alkyl-substituted butyrolactones have both inhibitory and stimulatory effects on GABA(A) receptors. Lactones with small alkyl substitutions at the alpha-position positively modulate the channel, whereas beta-substituted lactones tend to inhibit the GABA(A) receptor. These compounds mediate inhibition through the picrotoxin site
Hagai Tavori et al.
Bioorganic & medicinal chemistry, 16(15), 7504-7509 (2008-06-24)
Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters and lactones, whereas the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務