推薦產品
品質等級
化驗
99%
bp
310 °C (lit.)
mp
68-70 °C (lit.)
官能基
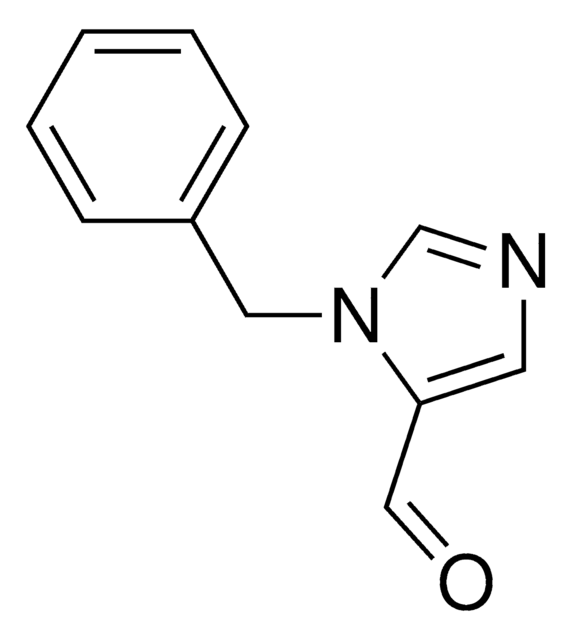
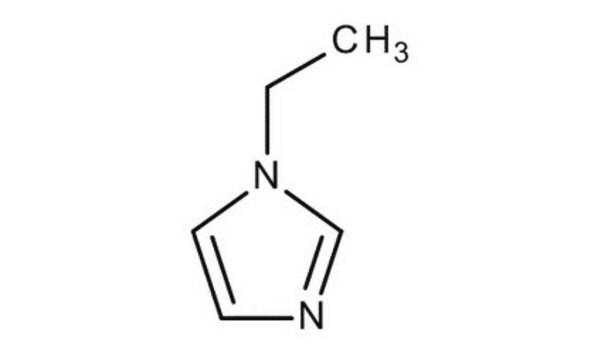
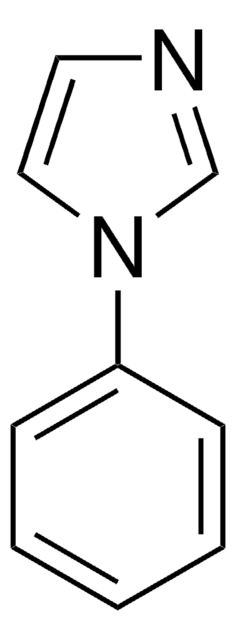
phenyl
SMILES 字串
C(c1ccccc1)n2ccnc2
InChI
1S/C10H10N2/c1-2-4-10(5-3-1)8-12-7-6-11-9-12/h1-7,9H,8H2
InChI 密鑰
KKKDZZRICRFGSD-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
1-苄基咪唑已被用于制备环糊精-离子液体聚合物(βCD-BIMOTs-TDI)。
生化/生理作用
1-苄咪唑是一种 CYP 抑制剂,可抑制鱼类中 MeO-BDEs(甲氧基-溴代二苯醚)生物转化为 OH-BDEs(羟基化)。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Jérémie Doiron et al.
European journal of medicinal chemistry, 46(9), 4010-4024 (2011-06-28)
A series of bis- and mono-benzonitrile or phenyl analogues of letrozole 1, bearing (1,2,3 and 1,2,5)-triazole or imidazole, were synthesized and screened for their anti-aromatase activities. The unsubstituted 1,2,3-triazole 10a derivative displayed inhibitory activity comparable with that of the aromatase
José María Navas et al.
Environmental toxicology and chemistry, 22(4), 830-836 (2003-04-11)
Xenobiotics can induce cytochrome P4501A (CYP1A) by ligand binding to the aryl hydrocarbon receptor (AhR). Typical AhR ligands are polycyclic aromatic compounds with planar molecular conformation. The present work investigated the ability of the N-imidazole derivative, 1-benzylimidazole (BIM), to induce
P Rothenbach et al.
Journal of applied physiology (Bethesda, Md. : 1985), 83(2), 530-536 (1997-08-01)
This study examines the hypothesis that intestinal ischemia-reperfusion (I/R) injury contributes to renal dysfunction by altered renal eicosanoid release. Anesthetized Sprague-Dawley rats underwent 60 min of sham or superior mesenteric artery (SMA) occlusion with 60 min of reperfusion. The I/R
A Grothusen et al.
Archives of toxicology, 71(1-2), 64-71 (1996-01-01)
Liver microsomes are a frequently used probe to investigate the phase I metabolism of xenobiotics in vitro. Structures containing nucleophilic hetero-atoms are possible substrates for cytochrome P450 enzymes (P450) and flavin-containing monooxygenases (FMO). Both enzymes are located in the endoplasmatic
Fredrik Jernerén et al.
Lipids, 47(7), 707-717 (2012-05-01)
(8R)-Hydroperoxy-(9Z,12Z)-octadecadienoic acid (8-HPODE) is formed by aspergilli as an intermediate in biosynthesis of oxylipins with effects on sporulation. 8-HPODE is transformed by separate diol synthases to (5S,8R)-dihydroxy- and (8R,11S)-dihydroxy-(9Z,12Z)-octadecadienoic acids (5,8- and 8,11-DiHODE). The former is formed by the cytochrome
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務

![3,6-二(2-噻吩基)-2,5-二氢吡咯并[3,4-c]吡咯-1,4-二酮 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)