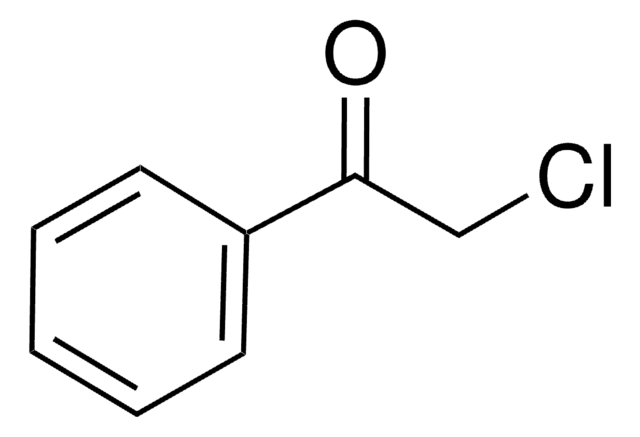
推薦產品
化驗
98%
形狀
solid
bp
135 °C/18 mmHg (lit.)
mp
48-51 °C (lit.)
官能基
bromo
SMILES 字串
BrCC(=O)c1ccccc1
InChI
1S/C8H7BrO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
InChI 密鑰
LIGACIXOYTUXAW-UHFFFAOYSA-N
基因資訊
human ... PTPN6(5777)
尋找類似的產品? 前往 產品比較指南
應用
2-溴苯乙酮已被用于分析有机酸,包括苯酰衍生物的形成。
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
客戶也查看了
J G Streeter
Plant physiology, 85(3), 768-773 (1987-11-01)
Metabolites in Bradyrhizobium japonicum bacteroids and in Glycine max (L.) Merr. cytosol from root nodules were analyzed using an isolation technique which makes it possible to estimate and correct for changes in concentration which may occur during bacteroid isolation. Bacteroid
P A Wender et al.
Organic letters, 1(13), 2117-2120 (2000-06-03)
[formula: see text] 4'-Bromoacetophenone derivatives which upon excitation can generate monophenyl radicals capable of hydrogen atom abstraction were investigated as photoinducible DNA cleaving agents. Pyrrolecarboxamide-conjugated 4'-bromoacetophenones were synthesized, and their DNA cleaving activities and sequence selectivities were determined.
Gulnur Arabaci et al.
Bioorganic & medicinal chemistry letters, 12(21), 3047-3050 (2002-10-10)
A series of alpha-haloacetophenone derivatives was tested for inhibition of protein tyrosine phosphatases SHP-1 and PTP1B. The results show that the bromides are much more potent than the corresponding chlorides, whereas the phenyl ring is remarkably tolerant to modifications. Derivatization
T Endoh et al.
Carcinogenesis, 17(3), 467-475 (1996-03-01)
Effects of inhibitors of arachidonic acid (AA) metabolism on the development of fatty liver, cirrhosis, glutathione-S-transferase placental form (GST-P)-positive nodules and the generation of 8-hydroxydeoxyguanosine (8-OHdG) and thiobarbituric acid-reactive substances (TBARS), caused by a choline-deficient, L-amino acid-defined (CDAA) diet, were
Mostafa A Hussein et al.
Acta pharmaceutica (Zagreb, Croatia), 59(4), 365-382 (2009-11-19)
5-Acyl-8-hydroxyquinoline-2-(3'-substituted-4'-aryl-2,3-dihydrothiazol-2'-ylidene)hydrazones, 5a-e to 10a-c, were prepared by the reaction of appropriate 5-acyl-8-hydroxyquinoline-4-substituted thiosemicarbazones 3a-e and phenacyl bromides 4a-e. Structures of the new compounds were verified on the basis of spectral and elemental analyses. Twenty-eight new compounds were tested for their
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務