V0882
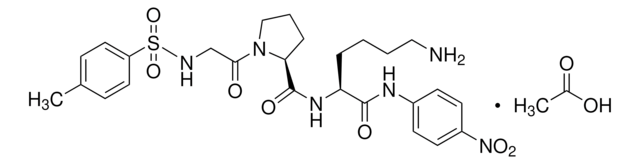
D-Val-Leu-Lys 4-nitroanilide dihydrochloride
plasmin substrate
Synonym(s):
D-Val-Leu-Lys-pNA dihydrochloride, D-Valyl-L-leucyl-L-lysine 4-nitroanilide dihydrochloride
About This Item
Recommended Products
Quality Level
Assay
≥98% (TLC)
form
powder
solubility
water: 50 mg/mL, clear, colorless to yellow
storage temp.
−20°C
SMILES string
Cl.Cl.CC(C)C[C@H](NC(=O)[C@H](N)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)Nc1ccc(cc1)[N+]([O-])=O
InChI
1S/C23H38N6O5.2ClH/c1-14(2)13-19(28-23(32)20(25)15(3)4)22(31)27-18(7-5-6-12-24)21(30)26-16-8-10-17(11-9-16)29(33)34;;/h8-11,14-15,18-20H,5-7,12-13,24-25H2,1-4H3,(H,26,30)(H,27,31)(H,28,32);2*1H/t18-,19-,20+;;/m0../s1
InChI key
VESQMNNSPPEOSZ-ZLARAOTRSA-N
Looking for similar products? Visit Product Comparison Guide
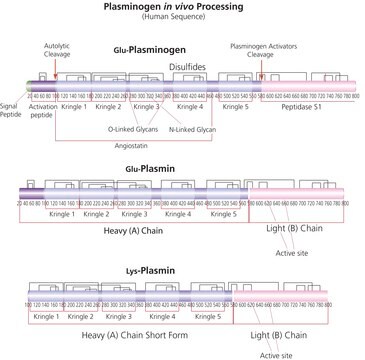
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
Protocol for Enzymatic Assay of Plasmin with D-Val-Leu-Lys-p-Nitroanilide Dihydrochloride
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service