SHP001
MISSION® Lentiviral Packaging Mix
Production of replication incompetent viral particles
Synonym(s):
Lentiviral Packaging, Lentiviral Packaging Kit
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41106609
NACRES:
NA.51
Recommended Products
Related Categories
General description
The MISSION® shRNA product line is centered on a transfer vector-based RNAi library against annotated mouse and human genes that can be used in conjunction with this Lentiviral Packaging Mix. In this packaging system, the pseudo-typing with VSV-G broadens the viral tropism associated with the virus. Therefore, these lentiviral particles can efficiently deliver the transfer sequence of interest to a wide variety of cell types, including primary and non-dividing cells.
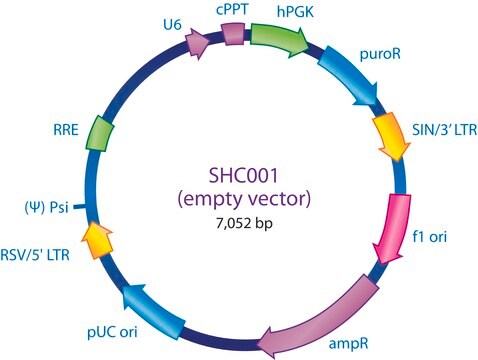
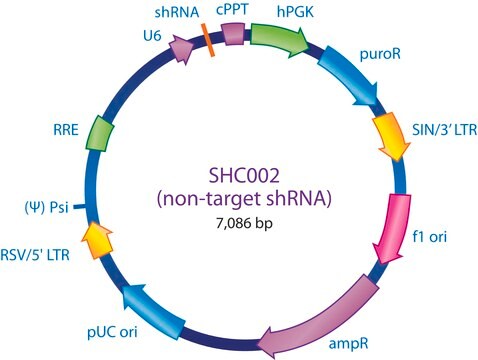
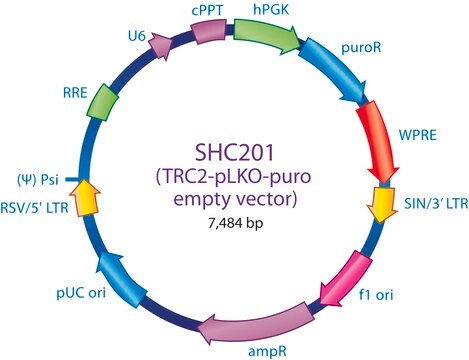
The MISSION® Lentiviral Packaging Mix is an optimized formulation of two plasmids expressing the key HIV packaging genes and a heterologous viral envelope gene.
Lentiviral particles are generated from three main components:
The Lentiviral Packaging Mix contains the first two components; it is designed to be co-transfected along with a compatible lentiviral transfer vector in order to create high-titer pseudo-typed lentiviral particles used for downstream transduction applications.
The MISSION® Lentiviral Packaging Mix is an optimized formulation of two plasmids expressing the key HIV packaging genes and a heterologous viral envelope gene.
Lentiviral particles are generated from three main components:
- The packaging vector, which contains the minimal set of lentiviral genes required to generate the virion structural proteins and packaging functions.
- The vesicular stomatitis virus G-protein envelope vector, which provides the heterologous envelope for pseudotyping.
- The shRNA transfer vector, which contains the sequence of interest as well as the cis acting sequences necessary for RNA production and packaging.
The Lentiviral Packaging Mix contains the first two components; it is designed to be co-transfected along with a compatible lentiviral transfer vector in order to create high-titer pseudo-typed lentiviral particles used for downstream transduction applications.
Application
MISSION® Lentiviral Packaging Mix has been used to incubate plasmids before addition to human embryonic kidney cells 293 expressing the large T antigen (293T cells) and to pack lentivirus into 293T cells.
Other Notes
Lentiviral particles are produced using a third-generation packaging system, which has many features that lead to enhanced biosafety. Recombinant lentiviruses produced with the MISSION Lentiviral Packaging Mix have not been shown to produce replication competent viral particles because of designed safety features. Users should consult and observe their own institutional guidelines before working with such viral systems.
Legal Information
Use of this product is subject to one or more license agreements. For details, please see http://sigmaaldrich.com/missionlicense .
MISSION is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
NIH Guidelines for Research Involving Recombinant DNA Molecules
N.I.H. Memorandum (2002)
T Dull et al.
Journal of virology, 72(11), 8463-8471 (1998-10-10)
Vectors derived from human immunodeficiency virus (HIV) are highly efficient vehicles for in vivo gene delivery. However, their biosafety is of major concern. Here we exploit the complexity of the HIV genome to provide lentivirus vectors with novel biosafety features.
Toufik Abbas-Terki et al.
Human gene therapy, 13(18), 2197-2201 (2003-01-25)
RNA interference (RNAi) is a form of posttranscriptional gene silencing mediated by short double-stranded RNA, known as small interfering RNA (siRNA). These siRNAs are capable of binding to a specific mRNA sequence and causing its degradation. The recent demonstration of
Wenwen Chien et al.
Journal of Cancer, 9(24), 4762-4773 (2018-12-28)
This study is an unbiased genomic screen to obtain functional targets for increased effectiveness of dasatinib in pancreatic cancer. Dasatinib, a multi-targeted tyrosine kinase inhibitor, is used in clinical trials for treatment of pancreatic cancer; however, intrinsic and acquired resistance
Helen He et al.
Journal of biomarkers, 2016, 1274603-1274603 (2016-06-02)
Objective. Use of tyramide signal amplification (TSA) to detect autophagy biomarkers in formalin fixed and paraffin embedded (FFPE) xenograft tissue. Materials and Methods. Autophagy marker regulation was studied in xenograft tissues using Amp HQ IHC and standard IHC methods. Results.
Articles
MISSION® Lentiviral shRNA
Protocols
Preparation of the Lentiviral Transduction Particles Using Packaging Plasmid Mix
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service