SCP0108
Cathepsin B Substrate
≥95% (HPLC), lyophilized
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C26H36N10O6
Molecular Weight:
584.63
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
Product Name
Cathepsin B Substrate, colorimetric,
Assay
≥95% (HPLC)
form
lyophilized
composition
Peptide Content, ≥80%
storage condition
protect from light
storage temp.
−20°C
Related Categories
Amino Acid Sequence
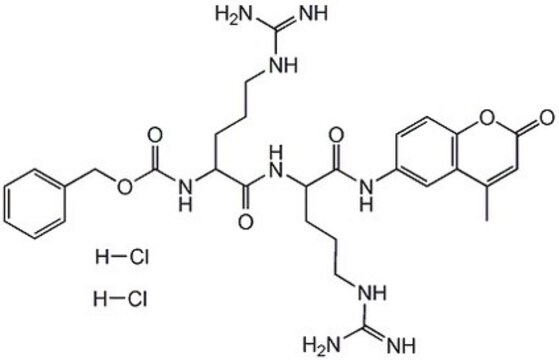
Z-Arg-Arg-pNA
General description
Cathepsin B, a bilobal protein, belongs to the papain-like family of cysteine proteases. It is produced as a preproenzyme. Its catalytic site is present at the interface between the two lobes. Cathepsin B has an occluding loop. It is located in the secretory vesicles of the neuronal cells. Active cathepsin B is found in the endosomal or lysosomal compartment under normal physiological conditions.
Biochem/physiol Actions
Cathepsin B is a lysosomal cysteine proteinase that metabolizes important molecules such as β-amyloid precursor protein into harmless fragments. Cathepsin B may be detected using the chromogenic substrate Z-Arg-Arg-pNA (z-arg-arg-p-nitroanalide) or flourogenic substrate Z-Arg-Arg-AMC (z-Arg-Arg-amino-4-methylcoumarin).
Cathepsin B possesses endopeptidase and exopeptidase activity. Active cathepsin B is mostly engaged in intracellular and extracellular protein turnover, which helps cells maintain homeostatic metabolic activity. It also participates in the regulation of pro-hormone and pro-enzyme activation, antigen processing, inflammatory reactions against antigens, tissue remodeling, and apoptosis. Cathepsin B plays a key role in acute pancreatitis. It also plays a vital role in lipid metabolism in atherosclerosis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cathepsin B: Basis Sequence: Mouse.
Dora Cavallo-Medved et al.
The AFCS-nature molecule pages, 2011 (2011-01-01)
S Hasnain et al.
The Journal of biological chemistry, 268(1), 235-240 (1993-01-05)
The pH dependence of cathepsin B-catalyzed hydrolyzes is very complex. At least seven dissociable groups are involved in the binding and hydrolysis of 7-amido-4-methyl coumarin and p-nitroaniline (pNA)-based substrates containing a P1 Arg and either a Phe or Arg at
Yasuhito Sako et al.
Experimental parasitology, 127(3), 693-701 (2010-11-26)
Cysteine peptidases have potent activities in the pathogenesis of various parasitic infections, and are considered as targets for chemotherapy and antigens for vaccine. In this study, two cathepsin B-like cysteine peptidases (EmCBP1 and EmCBP2) from Echinococcus multilocularis metacestodes were identified
Ilaria Giusti et al.
Neoplasia (New York, N.Y.), 10(5), 481-488 (2008-05-14)
Vesicles shed by cancer cells are known to mediate several tumor-host interactions. Tumor microenvironment may, in turn, influence the release and the activity of tumor-shed microvesicles. In this study, we investigated the molecular mediators of the pH-dependent proinvasive activity of
Angela J Eykelbosh et al.
Comparative biochemistry and physiology. Part A, Molecular & integrative physiology, 156(2), 218-223 (2010-02-23)
This study presents evidence that cathepsin B, a lysosomal protease, may be involved in the regulation of apoptosis during serum-starvation in teleost follicles. Zebrafish vitellogenic follicles were isolated, incubated under serum-free conditions and homogenized. The follicle extracts demonstrated caspase-3-like activity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service