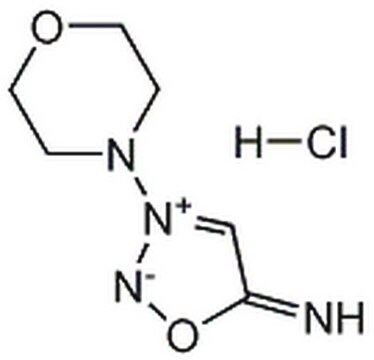
M5793
3-Morpholinosydnonimine hydrochloride
(consistent with structure, NMR)
Synonym(s):
3-(4-Morpholinyl)sydnone imine hydrochloride, Linsidomine hydrochloride, SIN-1 hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H10N4O2 · HCl
CAS Number:
Molecular Weight:
206.63
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
storage temp.
−20°C
SMILES string
Cl[H].[NH-]c1c[n+](no1)N2CCOCC2
InChI
1S/C6H10N4O2.ClH/c7-6-5-10(8-12-6)9-1-3-11-4-2-9;/h5,7H,1-4H2;1H
InChI key
NCGICGYLBXGBGN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Morpholinosydnonimine hydrochloride has been used:
- as a peroxynitrite donor standard to study the accumulation of peroxynitrite in Arabidopsis thaliana
- to study its effects on nitrosative stress in human brain vascular pericytes and human embryonic kidney cells
- as a ROS to study its effects on mouse embryonic fibroblasts
Biochem/physiol Actions
3-Morpholinosydnonimine participates in the inhibition of cysteine proteases.
Liberates nitric oxide (NO) spontaneously when in solution, activating guanylyl cyclase and causing an increase in cyclic-GMP. This product is a vasodilator and inhibits platelet aggregation. Using molecular oxygen, it generates both superoxide anion and nitric oxide that spontaneously form peroxynitrite.
Liberates nitric oxide (NO) spontaneously when in solution, activating guanylyl cyclase and causing an increase in cyclic-GMP; vasodilator.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Frank Gaupels et al.
Nitric oxide : biology and chemistry, 25(2), 222-228 (2011-02-08)
Nitric oxide (NO) is synthesized in plants in response to stress, and its role in signaling is well-documented. In contrast, very little is known about the physiological role of its derivate peroxynitrite (ONOO(-)), which forms when NO reacts with O(2)(-)
Keren Zohar et al.
Biomedicines, 9(9) (2021-09-29)
A hallmark of the aging brain is the robust inflammation mediated by microglial activation. Pathophysiology of common neurodegenerative diseases involves oxidative stress and neuroinflammation. Chronic treatment of aging rats by ladostigil, a compound with antioxidant and anti-inflammatory function, prevented microglial
H de Groot et al.
FEBS letters, 315(2), 139-142 (1993-01-04)
SIN-1 which spontaneously decomposes to yield nitric oxide (NO.) and superoxide anion (O2.-) radicals caused a loss of microsomal alpha-tocopherol paralleled by the formation of alpha-tocopheryl quinone. The loss was partially prevented by superoxide dismutase but not by catalase. The
Kittipot Sirichaiwetchakoon et al.
BioMed research international, 2020, 4183643-4183643 (2020-10-09)
Tea is one of the most popular beverages in the world. Camellia sinensis tea (CST) or green tea is widely regarded as a potent antioxidant. In Thailand, Pluchea indica (L.) Less. tea (PIT) has been commercially available as a health-promoting
Mushfiquddin Khan et al.
Journal of neuroinflammation, 8, 78-78 (2011-07-08)
Traumatic brain injury (TBI) induces primary and secondary damage in both the endothelium and the brain parenchyma, collectively termed the neurovascular unit. While neurons die quickly by necrosis, a vicious cycle of secondary injury in endothelial cells exacerbates the initial
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service