I5784
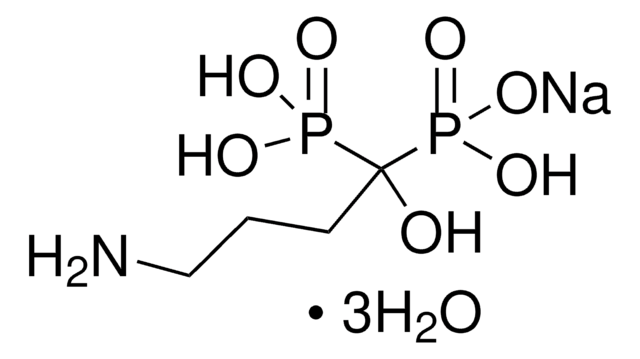
Ibandronate sodium salt
≥97% (NMR), solid
Synonym(s):
(1-Hydroxy-3-(methylpentylamino)propylidene)bisphosphonic acid sodium, Bondronat
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H22NNaO7P2
CAS Number:
Molecular Weight:
341.21
MDL number:
UNSPSC Code:
41106300
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥97% (NMR)
form
solid
storage condition
protect from light
color
white
solubility
H2O: >10 mg/mL
originator
Roche
storage temp.
2-8°C
SMILES string
[Na+].CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)([O-])=O
InChI
1S/C9H23NO7P2.Na/c1-3-4-5-7-10(2)8-6-9(11,18(12,13)14)19(15,16)17;/h11H,3-8H2,1-2H3,(H2,12,13,14)(H2,15,16,17);/q;+1/p-1
InChI key
LXLBEOAZMZAZND-UHFFFAOYSA-M
Gene Information
human ... FDPS(2224)
Application
Ibandronate sodium salt has been used to study its effect on the proliferation and ultrastructure of Leishmania and Giardia by the generation of concentration curves. It has also been used to elucidate the route by which nitrogen-containing bisphosphonates (N-BPs) enter the cytosol and inhibit their molecular target.
Biochem/physiol Actions
Ibandronate is a nitrogen-containing bisphosphonate (N-BP). It potentially inhibits mevalonate pathway in osteoclasts. Thus, ibandronate is effectively used to treat osteoporosis and other bone-related diseases.
Ibandronate sodium inhibits farnesyl diphosphate synthase (IC50 = 20 nM). Ibandronate sodium is also a bone resorption inhibitor. It has been investigated for in vitro anti-tumor effects, such as apoptosis induction, inhibitor of cell growth, inhibition of invasive behavior, and inhibition of angiogenesis and for its in vivo role in various cancers including breast and prostate cancers.
Features and Benefits
This compound was developed by Roche. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Insights about the structure of farnesyl diphosphate synthase (FPPS) and the activity of bisphosphonates on the proliferation and ultrastructure of Leishmania and Giardia
Gadelha APR, et al.
Parasites & vectors, 13, 1-18 (2020)
Ricardo Villa-Bellosta et al.
Arteriosclerosis, thrombosis, and vascular biology, 29(5), 761-766 (2009-02-14)
The role of inorganic phosphate in the pathogenesis of vascular calcification (VC) has been studied extensively in recent years. Phosphonoformic acid (PFA), an inhibitor of type II Pi transporters, has been traditionally used to study the involvement of Pi transport
Ana Paula R Gadelha et al.
Parasites & vectors, 13(1), 168-168 (2020-04-07)
The enzyme farnesyl diphosphate synthase (FPPS) is positioned in the intersection of different sterol biosynthesis pathways such as those producing isoprenoids, dolichols and ergosterol. FPPS is ubiquitous in eukaryotes and is inhibited by nitrogen-containing bisphosphonates (N-BP). N-BP activity and the
Manish Rauthan et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(15), 5981-5986 (2013-03-27)
Statins are cholesterol-lowering drugs that inhibit 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, the rate-limiting enzyme in the synthesis of cholesterol via the mevalonate pathway. This pathway also produces coenzyme Q (a component of the respiratory chain), dolichols (important for protein glycosylation), and isoprenoids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service