G2536
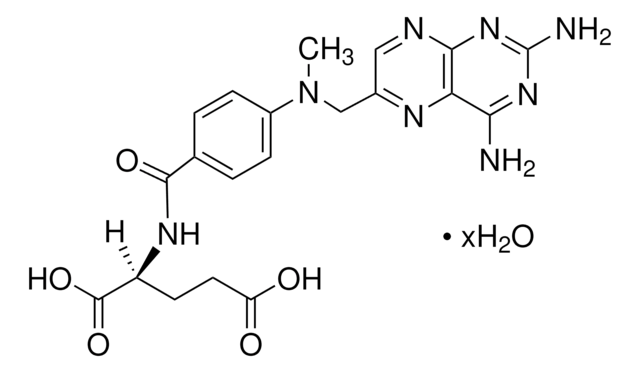
Ganciclovir
≥99% (HPLC), powder, viral DNA elongation inhibitor
Synonym(s):
2-Amino-1,9-dihydro-9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-6H-purin-6-one, 9-(1,3-Dihydroxy-2-propoxymethyl)guanine, DHPG
About This Item
Recommended Products
product name
Ganciclovir, ≥99% (HPLC), powder
Quality Level
Assay
≥99% (HPLC)
form
powder
color
white
solubility
0.1 M HCl: 10 mg/mL
ε (extinction coefficient)
12.0 at 256 nm at 1 mM
antibiotic activity spectrum
viruses
Mode of action
DNA synthesis | interferes
originator
Roche
storage temp.
2-8°C
SMILES string
NC1=Nc2c(ncn2COC(CO)CO)C(=O)N1
InChI
1S/C9H13N5O4/c10-9-12-7-6(8(17)13-9)11-3-14(7)4-18-5(1-15)2-16/h3,5,15-16H,1-2,4H2,(H3,10,12,13,17)
InChI key
IRSCQMHQWWYFCW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Upon expression of a viral suicide gene encoding thymidine kinase, the non-toxic pro-drug is converted to a phosphorylated active analog and is incorporated into the DNA of replicating eukaryotic cells, causing death of the malignant dividing cell. The cell cycle is irreversibly arrested at the G2-M checkpoint. Gap junction involvement in the ganciclovir bystander effect has been studied. Ganciclovir has been used to study loss of telomeres and to evaluate sensitivity of viruses to antiviral treatments.
Features and Benefits
Preparation Note
This product should be stored desiccated at 2-8 °C. Under these conditions the product is stable for 3 years.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 1B - Repr. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
MISSION® Target ID Library for Human miRNA Target Identification and Discovery;
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service