F124
Furafylline
≥98% (HPLC), powder, caffeine inhibitor
Synonym(s):
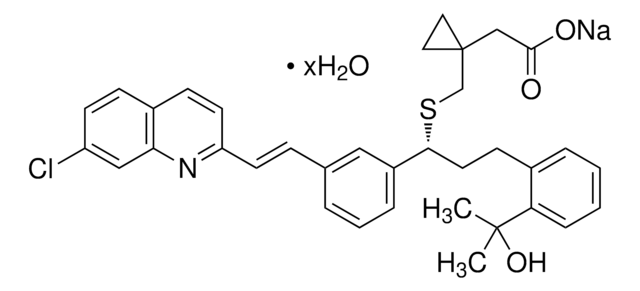
3-(2-Furanylmethyl)-3,7-dihydro-1,8-dimethyl-1H-purine-2,6-dione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H12N4O3
CAS Number:
Molecular Weight:
260.25
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Product Name
Furafylline, ≥98% (HPLC)
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to beige
mp
274-275 °C
solubility
DMSO: 10 mg/mL, clear
storage temp.
room temp
SMILES string
CN1C(=O)N(Cc2ccco2)c3nc(C)[nH]c3C1=O
InChI
1S/C12H12N4O3/c1-7-13-9-10(14-7)16(6-8-4-3-5-19-8)12(18)15(2)11(9)17/h3-5H,6H2,1-2H3,(H,13,14)
InChI key
KGQZGCIVHYLPBH-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Biochem/physiol Actions
Furafylline (1,8-dimethyl-3-(2′ -furfuryl)methylxanthine) is a xanthine derivative. It is preferred in treating asthma. It serves as a N3-demethylation inhibitor of caffeine. Furafylline does not show much effect on human monooxygenase activities. It is considered as an efficient bronchodilator and as an inhibitor of anaphylactic reactions, when compared to theophylline.
Furafylline is a methyl xanthine derivative with longer duration of action than theophylline and an inhibitor of cytochrome P4501A2.
Features and Benefits
This compound is a featured product for ADME Tox and Cyclic Nucleotide research. Discover more featured ADME Tox and Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Robert J Turesky et al.
Mutation research, 506-507, 187-195 (2002-09-28)
The metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) was investigated in primary human and rat hepatocytes. The genotoxic metabolites 2-(hydroxyamino)-3,8-dimethylimidazo[4,5-f]quinoxaline (HONH-MeIQx) and 2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine (HONH-PhIP), which are formed by cytochrome P4501A2 (CYP1A2), were detected as stable N(2)-glucuronide and N(2)- and N(3)-glucuronide
Lars Brachtendorf et al.
Pharmacology & toxicology, 90(3), 144-149 (2002-06-20)
From case reports of patients treated with the tetracyclic antidepressant drug maprotiline, it appears that this drug is subject to polymorphic metabolism. Thus, we studied formation of the major maprotiline metabolite desmethylmaprotiline to identify the human cytochrome P-450 enzymes (CYP)
X Boulenc et al.
The Journal of pharmacology and experimental therapeutics, 263(3), 1471-1478 (1992-12-01)
The expression and inducibility of cytochrome P450IA1 isozyme was investigated in the human carcinoma cell line Caco-2 cultured between days 7 and 35 in the absence or the presence of various enzyme inducers such as 3-methylcholanthrene, beta-naphthoflavone (beta NF), dioxin
Jinlan Wei et al.
Pharmaceutical biology, 56(1), 363-367 (2018-08-21)
Friedelin is a triterpenoid with several biological activities. However, the affects of Friedelin on the activity of human liver cytochrome P450 (CYP) enzymes remains unclear. This study investigates the inhibitory effects of Friedelin on the major human liver CYP isoforms
Furafylline is a potent and selective inhibitor of cytochrome P450IA2 in man.
Sesardicm D, et al.
British Journal of Clinical Pharmacology, 29(6), 651-663 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service