D1771
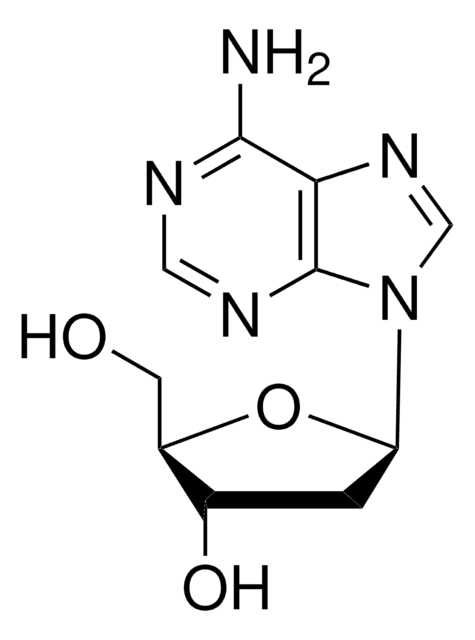
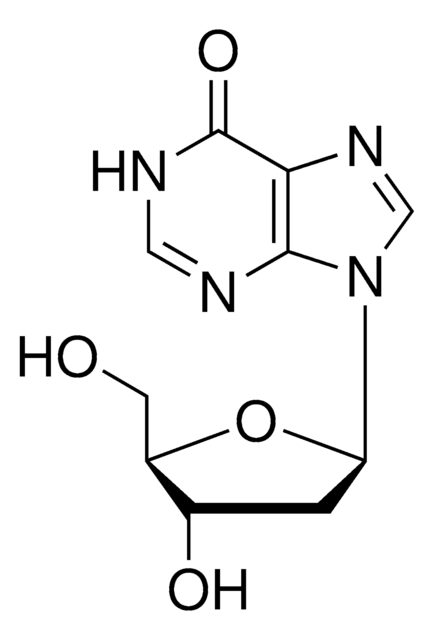
5′-Deoxyadenosine
methylthioadenosine/S-adenosylhomocysteine (MTA/SAH) nucleosidase substrate
Synonym(s):
5′-dAdo
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H13N5O3
CAS Number:
Molecular Weight:
251.24
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98% (TLC)
form
powder
solubility
hot water: 19.60-20.40 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C10H13N5O3/c1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15/h2-4,6-7,10,16-17H,1H3,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1
InChI key
XGYIMTFOTBMPFP-KQYNXXCUSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5′-Deoxyadenosine has been used:
- as a standard in mass spectroscopy
- as an inhibitor for screening thymidine phosphorylase activity
- as a substrate in 5′-Deoxyadenosine deaminase (DadD) assay
Biochem/physiol Actions
5′-Deoxyadenosine is a substrate for the enzyme methylthioadenosine/S-adenosylhomocysteine (MTA/SAH) nucleosidase in microbes. 5′-Deoxyadenosine is a byproduct of cleavage of S-adenosylmethionine (SAM). High levels of 5′-Deoxyadenosine inhibits SAM dependent enzymes. It also inhibits biotin synthase (BioB) and lipoyl synthase (LipA) enzymes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The nucleoside derivative 5?-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action
Liekens S, et al.
Test, 279(28), 29598-29605 (2004)
Kenichi Yokoyama et al.
Biochemistry, 47(34), 8950-8960 (2008-08-05)
BtrN is a radical SAM ( S-adenosyl- l-methionine) enzyme that catalyzes the oxidation of 2-deoxy- scyllo-inosamine (DOIA) into 3-amino-2,3-dideoxy- scyllo-inosose (amino-DOI) during the biosynthesis of 2-deoxystreptamine (DOS) in the butirosin producer Bacillus circulans. Recently, we have shown that BtrN catalyzes
Charles J Walsby et al.
Inorganic chemistry, 44(4), 727-741 (2005-04-30)
Electron paramagnetic resonance (EPR), electron-nuclear double resonance (ENDOR), and Mössbauer spectroscopies and other physical methods have provided important new insights into the radical-SAM superfamily of proteins, which use iron-sulfur clusters and S-adenosylmethionine to initiate H atom abstraction reactions. This remarkable
Joseph T Jarrett
Current opinion in chemical biology, 7(2), 174-182 (2003-04-26)
Adenosylmethionine-dependent radical enzymes provide a novel mechanism for generating the highly oxidizing 5'-deoxyadenosyl radical in an anaerobic reducing environment. Recent studies suggest a unique covalent interaction between adenosylmethionine and a catalytic iron-sulfur cluster that may promote inner-sphere electron transfer to
Gunhild Layer et al.
Current opinion in chemical biology, 8(5), 468-476 (2004-09-29)
'Radical SAM' enzymes juxtapose a [4Fe-4S] cluster and S-adenosyl-l-methionine (SAM) to generate catalytic 5'-deoxyadenosyl radicals. The crystal structures of oxygen-independent coproporphyrinogen III oxidase HemN and biotin synthase reveal the positioning of both cofactors with respect to each other and relative
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service