B3503
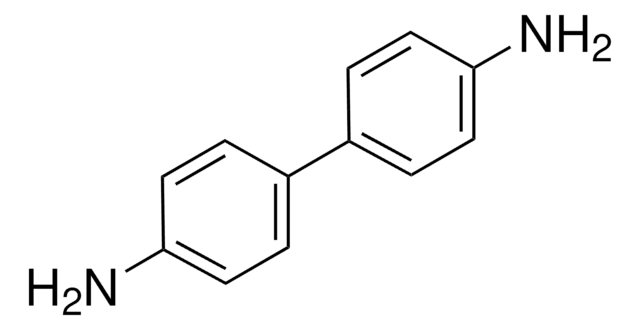
Benzidine
≥98.0% (N)
Synonym(s):
4,4′-Diaminobiphenyl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H12N2
CAS Number:
Molecular Weight:
184.24
Beilstein:
742770
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (N)
form
powder
SMILES string
Nc1ccc(cc1)-c2ccc(N)cc2
InChI
1S/C12H12N2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8H,13-14H2
InChI key
HFACYLZERDEVSX-UHFFFAOYSA-N
Gene Information
human ... UGT1A4(54657)
Looking for similar products? Visit Product Comparison Guide
Application
Benzidine (4,4′-Diaminobiphenyl) is an environmental genotoxin that posses increased risks of cancer to exposed people. Benzidine may be used as a reference material in procedures that analyze for benzidine. Benzidine can be used in studies on its mechanisms of genotoxicity.
Biochem/physiol Actions
Initiates tumors in the bladder under experimental conditions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Benzidine: a bladder carcinogen.
R G Wendel et al.
The Journal of urology, 111(5), 607-610 (1974-05-01)
Brian J Pak et al.
PLoS neglected tropical diseases, 8(8), e3002-e3002 (2014-08-08)
Strongyloidiasis is a persistent human parasitic infection caused by the intestinal nematode, Strongyloides stercoralis. The parasite has a world-wide distribution, particularly in tropical and subtropical regions with poor sanitary conditions. Since individuals with strongyloidiasis are typically asymptomatic, the infection can
G Choudhary
Chemosphere, 32(2), 267-291 (1996-01-01)
Benzidine, an odorless, white to slightly reddish-white crystalline organic compound, is an environmental contaminant that has been identified at about 30 National Priorities List (NPL) hazardous waste sites in the United States. In the environment, it is usually found attached
P D Josephy
Federation proceedings, 45(10), 2465-2470 (1986-09-01)
Benzidine oxidative activation may proceed by peroxidase-catalyzed one-electron oxidation via free radical intermediates, or by N-acetylation followed by monooxygenase-catalyzed N-hydroxylation. The peroxidase route has been examined by using horseradish peroxidase or prostaglandin H synthase in vitro. In the presence of
J Whysner et al.
Pharmacology & therapeutics, 71(1-2), 107-126 (1996-01-01)
The aromatic amine benzidine (BZ) has produced various tumors, including liver tumors, in mice, rats and hamsters. BZ forms DNA adducts in rodent liver, and it is positive in most genotoxicity tests. Only bladder tumors are produced in dogs and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service