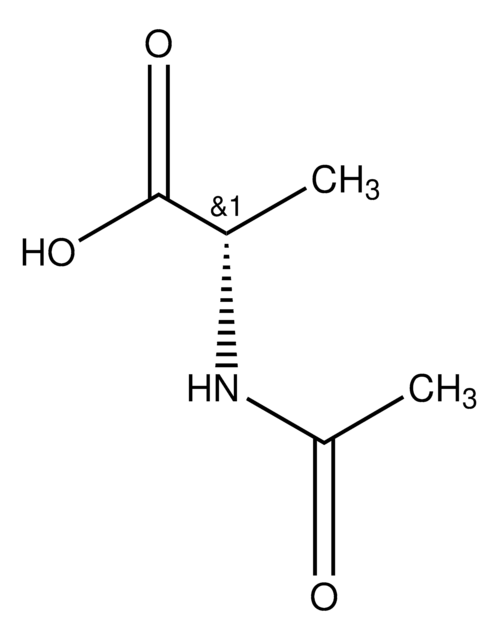
A1501
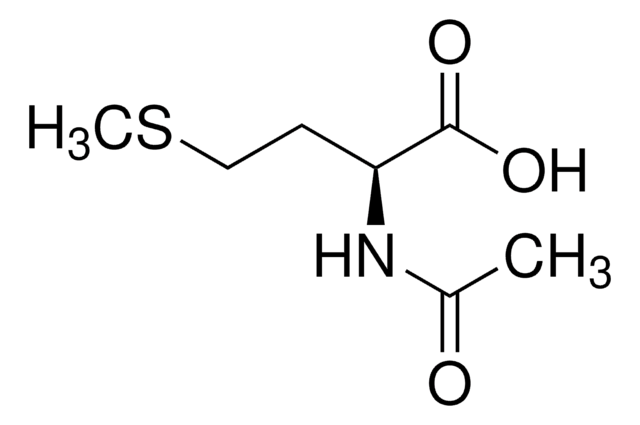
N-Acetyl-D-methionine
~99%, suitable for ligand binding assays
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H13NO3S
CAS Number:
Molecular Weight:
191.25
Beilstein:
1725553
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
N-Acetyl-D-methionine, ~99%
Assay
~99%
Quality Level
form
powder or crystals
technique(s)
ligand binding assay: suitable
color
white
mp
102.3-103.6 °C
storage temp.
−20°C
SMILES string
CSCC[C@@H](NC(C)=O)C(O)=O
InChI
1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)/t6-/m1/s1
InChI key
XUYPXLNMDZIRQH-ZCFIWIBFSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Acetyl-D-methionine may be used as a substrate to identify, differentiate and characterized N-acylamino acid racemase(s) and N-acyl-D-amino acid amidohydrolase(s).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wen-Ching Wang et al.
Journal of molecular biology, 342(1), 155-169 (2004-08-18)
N-acylamino acid racemase (NAAAR) catalyzes the racemization of N-acylamino acids and can be used in concert with an aminoacylase to produce enantiopure alpha-amino acids, a process that has potential industrial applications. Here we have cloned and characterized an NAAAR homologue
Pei-Hsun Lin et al.
European journal of biochemistry, 269(19), 4868-4878 (2002-10-02)
An N-acyl-d-amino acid amidohydrolase (N-D-AAase) was identified in cell extracts of a strain, Iso1, isolated from an environment containing N-acetyl-d-methionine. The bacterium was classified as Variovorax paradoxus by phylogenetic analysis. The gene was cloned and sequenced. The gene consisted of
S Pittelkow et al.
Protein expression and purification, 12(2), 269-276 (1998-03-31)
Aminoacylase I (EC 3.5.1.14) is one of the most abundant enzymes in the cortical region of mammalian kidney. Both the porcine and the human enzyme were overexpressed using baculovirus expression vector systems and purified by hydrophobic interaction chromatography and anion-exchange
T Odajima et al.
Cell biochemistry and function, 16(2), 139-147 (1998-06-24)
Urate oxidase from Candida utilis, an enzyme containing an essential thiol, was examined for its sensitivity to lactoperoxidase, an oxidant present in breast milk. Upon exposure to a system composed of lactoperoxidase, hydrogen peroxide and bromide at moderately alkaline pH
M J Wick et al.
Biochemical pharmacology, 37(7), 1225-1231 (1988-04-01)
Both N-hydroxy-2-acetamidofluorene (N-OH-AAF) and the heterocyclic analogue, 2-(N-hydroxyacetamido)carbazole (N-OH-AAC), were shown to be mechanism-based irreversible inhibitors (suicide inhibitors) of partially purified rat hepatic N-acetyltransferase (NAT) activity. Although N-OH-AAC exhibited an apparent first-order inactivation rate constant (ki) that was 7-fold lower
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service