NIST972A
Vitamin D metabolites in frozen human serum
NIST® SRM® 972a
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
certified reference material
Quality Level
form
liquid
packaging
pkg of 4 x 1 mL
manufacturer/tradename
NIST®
technique(s)
mass spectrometry (MS): suitable
application(s)
clinical testing
format
matrix material
General description
Determining total 25-hydroxy vitamin D [25(OH)D] concentration in serum, the sum of 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3] is generally considered a reliable indicator of vitamin D status. Measurement of 24R, 25(OH)2D3 in serum is considered a catabolism marker and an indicator of kidney disease. A unit of SRM 972a contains 1 mL of four vials (levels 1 through 4) of frozen serum with different concentration levels of 25-hydroxyvitamin D [25(OH)D] and 24R, 25-dihydroxy vitamin D3 [24R,25(OH)2D3].
NIST972A_Cert
NIST972A_SDS
NIST972A_Cert
NIST972A_SDS
Application
This vitamin D metabolite standard reference material (SRM) is intended for use as an accuracy control in the evaluation of methods for determining the amount-of-substance concentration of vitamin D metabolites in human serum. This SRM can also be used as a quality assurance tool for assigning values to in-house control materials for these constituents.
Vitamin D metabolites in frozen human serum has been used as a reference material for the calibration and standardization of 25-hydroxyvitamin D (25(OH)D) from dried blood spot samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Biochem/physiol Actions
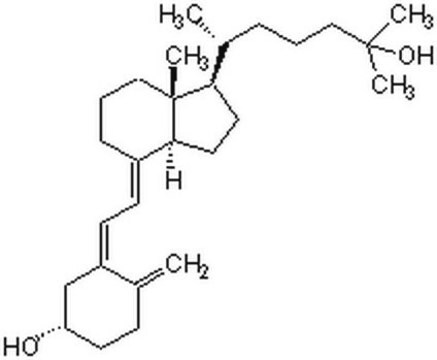
Vitamin D known as cholecalciferol exists as vitamin D3. It is taken in the diet through fortified dairy products and fish oils. It is synthesized in the skin from 7-dehydrocholesterol by ultraviolet irradiation. The biologically active form of vitamin D, 25(OH)2D3, is essential for mineral metabolism, and for other physiological functions like inhibition of growth of cancer cells and protection against certain immune mediated disorders.
Other Notes
- Information on expiry, storage, and usage is provided in the NIST certificate.
- Notes on Biomaterials, disposal, and transport are available on NIST MSDS.
Preparation Note
- The vials should be stored in the dark at a temperature between –20 °C and –80 °C.
- Before use, the vial/s must be thawed at room temperature for about 30 mins in subdued light.
Legal Information
NIST is a registered trademark of National Institute of Standards and Technology
SRM is a registered trademark of National Institute of Standards and Technology
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Mena-Bravo et al.
Journal of chromatography. A, 1451, 50-57 (2016-05-18)
A method based on automated on-line solid phase extraction coupled to two-dimensional liquid chromatography with tandem mass spectrometry detection (SPE-2DLC-MS/MS) is here reported for vitamin D metabolite profiling in human serum with absolute quantitation. Two-dimensional LC was configured with two
Carl Jenkinson et al.
Clinical chemistry and laboratory medicine, 59(10), 1642-1652 (2021-05-21)
Clinical evaluation of vitamin D status is conventionally performed by measuring serum levels of a single vitamin D metabolite, 25-hydroxyvitamin D predominantly by immunoassay methodology. However, this neglects the complex metabolic pathways involved in vitamin D bioactivity, including two canonical
Overestimation of 25-hydroxyvitamin D3 by increased ionisation efficiency of 3-epi-25-hydroxyvitamin D3 in LC-MS/MS methods not separating both metabolites as determined by an LC-MS/MS method for separate quantification of 25-hydroxyvitamin D3, 3-epi-25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum
van den Ouweland JMW, et al.
Journal of Chromatography. B, Biomedical Sciences and Applications, 967, 195-202 (2014)
Vitamin D: metabolism
Christakos S, et al.
Rheumatic Diseases Clinics of North America, 38(1), 1-11 (2012)
Simplified 25-hydroxyvitamin D standardization and optimization in dried blood spots by LC-MS/MS
Makowski AJ, et al.
Journal of AOAC (Association of Official Analytical Chemists) International, 100(5), 1328-1336 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service