A10701
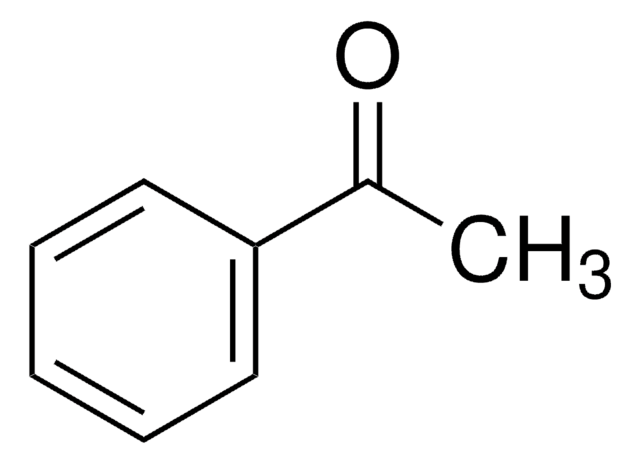
Acetophenone
ReagentPlus®, 99%
Synonym(s):
Methyl phenyl ketone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3COC6H5
CAS Number:
Molecular Weight:
120.15
Beilstein:
605842
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
4.1 (vs air)
Quality Level
vapor pressure
0.45 mmHg ( 25 °C)
1 mmHg ( 15 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
autoignition temp.
1058 °F
refractive index
n20/D 1.534 (lit.)
bp
202 °C (lit.)
mp
19-20 °C (lit.)
density
1.03 g/mL at 25 °C (lit.)
SMILES string
CC(=O)c1ccccc1
InChI
1S/C8H8O/c1-7(9)8-5-3-2-4-6-8/h2-6H,1H3
InChI key
KWOLFJPFCHCOCG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Acetophenone is an aromatic ketone used in the synthesis of alcohol by catalytic hydrogenation, and also in perfumery.
Acetophenone (AP, methyl phenyl ketone) is a common industrial solvent. It can be synthesized from 1-phenylethanol in the presence of PdAu-NPs-TiO2 (titanium dioxide-supported palladium gold bimetallic nanoparticles) hybrid. Its vacuum ultraviolet absorption spectrum shows absorption bands at 196, 191, 179 and 167mμ. AP reacts with α-naphtylphenylsilane in the presence of N-chelate ligands based on chiral oxazolines to undergo hydrosilylation with high enantioselectivity. It can also undergo hydrogenation reaction in the presence of different transition metal catalysts under different conditions.
Acetophenone (AP, methyl phenyl ketone) is a common industrial solvent. It can be synthesized from 1-phenylethanol in the presence of PdAu-NPs-TiO2 (titanium dioxide-supported palladium gold bimetallic nanoparticles) hybrid. Its vacuum ultraviolet absorption spectrum shows absorption bands at 196, 191, 179 and 167mμ. AP reacts with α-naphtylphenylsilane in the presence of N-chelate ligands based on chiral oxazolines to undergo hydrosilylation with high enantioselectivity. It can also undergo hydrogenation reaction in the presence of different transition metal catalysts under different conditions.
Application
Acetophenone was used to induce peak currents in M71 neurons using voltage clamp. It may be used in the synthesis of N-(1-phenylethyl)formamide, via Leuckart reaction.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solvent effects in heterogeneous selective hydrogenation of acetophenone: differences between Rh/C and Rh/Al2O3 catalysts and the superiority of water as a functional solvent.
Yoshida H, et al.
Green Chemistry, 17(3), 1877-1883 (2015)
Acetophenones with selective antimycobacterial activity.
Rajabi L, et al.
Letters in Applied Microbiology, 40(3), 212-217 (2005)
Photocatalytic reduction of acetophenone in membrane reactors under UV and visible light using TiO2 and Pd/TiO2 catalysts.
Molinari R, et al.
Chemical Engineering Journal, 274, 307-316 (2015)
Jiwei He et al.
The European journal of neuroscience, 36(4), 2452-2460 (2012-06-19)
Early experience considerably modulates the organization and function of all sensory systems. In the mammalian olfactory system, deprivation of the sensory inputs via neonatal, unilateral naris closure has been shown to induce structural, molecular and functional changes from the olfactory
Green-chemistry Compatible Approach to TiO2-supported PdAu Bimetallic Nanoparticles for Solvent-free 1-Phenylethanol Oxidation under Mild Conditions.
Chang JB, et al.
Nano-Micro Letters, 7(3), 307-315 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service