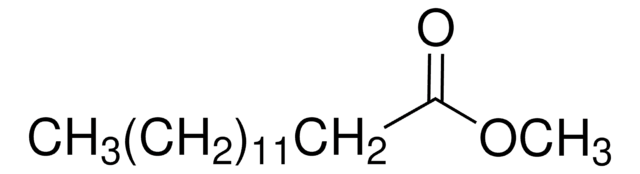70129
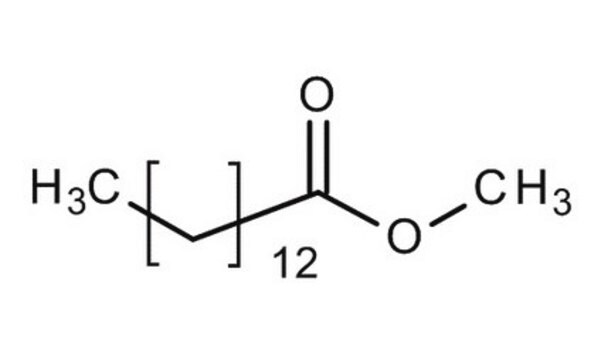
Methyl myristate
analytical standard
Synonym(s):
Methyl tetradecanoate, Myristic acid methyl ester
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥99.5% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.436 (lit.)
n20/D 1.438
bp
323 °C (lit.)
mp
18 °C (lit.)
density
0.855 g/mL at 25 °C (lit.)
format
neat
functional group
ester
shipped in
ambient
storage temp.
room temp
SMILES string
CCCCCCCCCCCCCC(=O)OC
InChI
1S/C15H30O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15(16)17-2/h3-14H2,1-2H3
InChI key
ZAZKJZBWRNNLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Comparative analysis of gas chromatography-combustion-mass spectrometry and gas chromatography-flame ionization detector methods for the determination of fatty acid methyl esters (FAMEs) in biodiesel samples
- Gas chromatography-tandem differential mobility spectrometry (DMS) based separation and quantification of 16 methyl- and ethyl- fatty acid esters from biodiesel samples
- Analysis of coffee oil and residue obtained from roasted coffee beans to determine the composition of 11 fatty acids following their methyl esterification by gas chromatography coupled with a flame ionization detector (GC-FID)
- Simultaneous determination of fatty acids in bovine colostrum samples by GC-FID after their derivatization to ester forms using an acidic catalyst boron trifluoride
- Separation and quantification of eight fatty acids after their derivatization to methyl esters in the oil extracted from the leaves of Abutilon hirtum (Lam.) by GC-MS
Other Notes
Recommended products
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112.0 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
-9,10-methylenehexadecanoate
Protocols
Separation of Methyl erucate; Methyl palmitate; Methyl stearate; Methyl linolenate; Methyl eicosenoate; Methyl behenate; Methyl myristate; Methyl oleate; Methyl arachidate
Separation of Methyl decanoate; Methyl dodecanoate; Methyl myristate; Methyl palmitate; Methyl caprylate; Methyl oleate; Methyl linoleate; Methyl linolenate; Methyl stearate
-11-eicosenoate; Methyl elaidate; Methyl linoleate; Methyl myristate; Methyl myristoleate; Methyl palmitate; Methyl palmitoleate; Methyl oleate; Methyl pentadecanoate; Methyl tridecanoate; Methyl behenate; Methyl caprylate; Methyl erucate; Methyl heptadecanoate; Methyl arachidate
gc-analysis-of-a-37-component-fame-mix-g004278
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service