8.52045
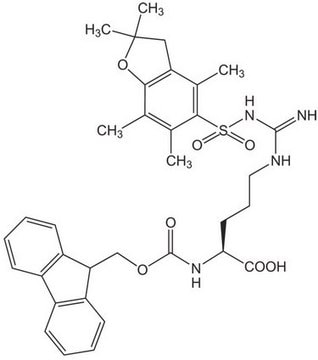
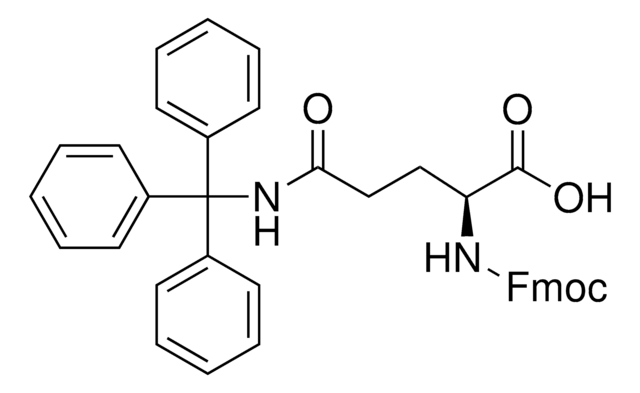
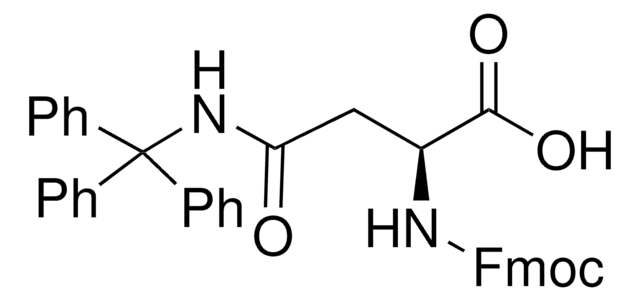
Fmoc-Gln(Trt)-OH
Novabiochem®
Synonym(s):
Fmoc-Gln(Trt)-OH, N-α-Fmoc-N-γ-trityl-L-glutamine
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
Assay
≥93.0% (acidimetric)
≥98% (TLC)
≥99.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
mp
110-125 °C
application(s)
peptide synthesis
functional group
amide
storage temp.
-10 to -25°C
InChI
1S/C39H34N2O5/c42-36(41-39(27-14-4-1-5-15-27,28-16-6-2-7-17-28)29-18-8-3-9-19-29)25-24-35(37(43)44)40-38(45)46-26-34-32-22-12-10-20-30(32)31-21-11-13-23-33(31)34/h1-23,34-35H,24-26H2,(H,40,45)(H,41,42)(H,43,44)/t35-/m0/s1
InChI key
WDGICUODAOGOMO-DHUJRADRSA-N
Related Categories
General description
Fmoc-Gln(Trt)-OH has good solubility properties in most organic solvents, and its use has been shown to result in significantly purer peptides than other derivatives used for the introduction of Gln [1]. Coupling can be performed by standard procedures. The trityl-protecting group is normally removed by 95% TFA in 1-3 hours, with no alkylation of Trp.
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] P. Sieber, et al. (1991) Tetrahedron Lett., 32, 739
Application
- Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design: This study highlights the use of Fmoc-Gln(Trt)-OH in the synthesis of peptide substrates for biochemical assays, contributing to the development of COVID-19 therapeutics (Rut et al., 2020).
Linkage
Analysis Note
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 160 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.8 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Gln (Trt) -OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Gln(Trt)-Gln(Trt)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Gln-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (GC): ≤ 0.2 %
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Solubility (25 mmole in 50 ml DMF): clearly soluble
Assay (acidimetric): ≥ 93.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service