1.14896
Iron Test, photometric
photometric, 1.0-50.0 mg/L (Fe), Spectroquant®
About This Item
Recommended Products
product name
Iron Cell Test, photometric, 1.0-50.0 mg/L (Fe), Spectroquant®
product line
Spectroquant®
Quality Level
usage
sufficient for 25 tests
specific analyte(s)
iron
measuring range
1.0-50.0 mg/L (Fe)
technique(s)
photometry: suitable
compatibility
for use with Spectroquant® Nova 60 A
for use with Spectroquant® Prove 100
for use with Spectroquant® Prove 300
for use with Spectroquant® Prove 600
detection method
photometric (2,2’-Bipyridine)
storage temp.
15-25°C
General description
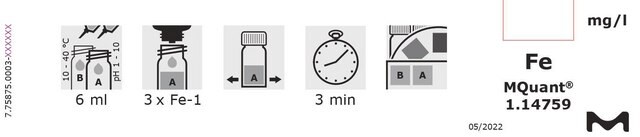
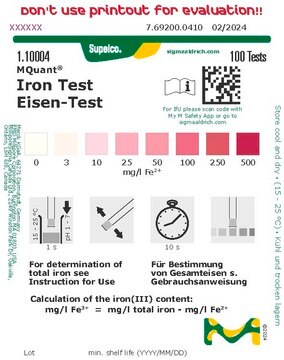
This Spectroquant® Iron test allows the accurate quantification of iron ions in various waters.All iron ions are reduced to iron(II) ions by ascorbic acid.
In a buffered medium these react with 2,2’-bipyridine to form a red complex that is determined photometrically. Without the addition of ascorbic acid (reagent Fe-1K), the test measures only iron(II).
MQuant® test strips can be used for pre-testing a sample in an economic and quick way to estimate the iron concentration and a possible dilution factor prior to using a quantitative method such as the Spectroquant® test.
All our Cell and Reagent Test Kits are equipped with the unique Live ID (2D barcode) which allows seamless method recognition and contains essential information such as lot number, expiry date, and automatic calibration updates.
The Spectroquant® Cell Tests come with prefilled 16 mm round cells as well as all the required reagents to perform the analysis according to the procedure described in the accompanying instruction leaflet.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
Photometric determination subsequent to acid mineralisation
Photometric determination subsequent to decomposition with sulfuric acid and Perhydrol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service