All Photos(1)
About This Item
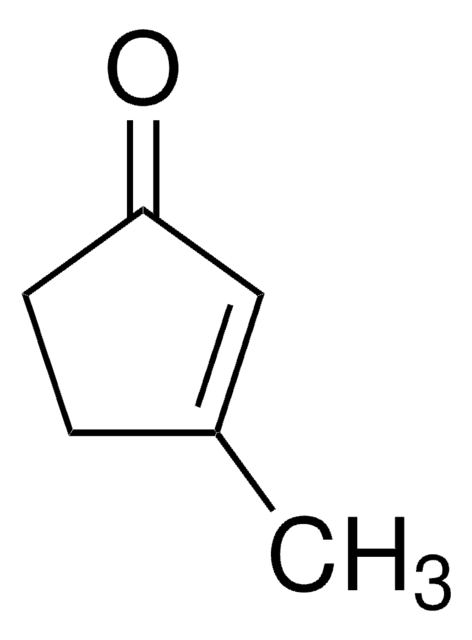
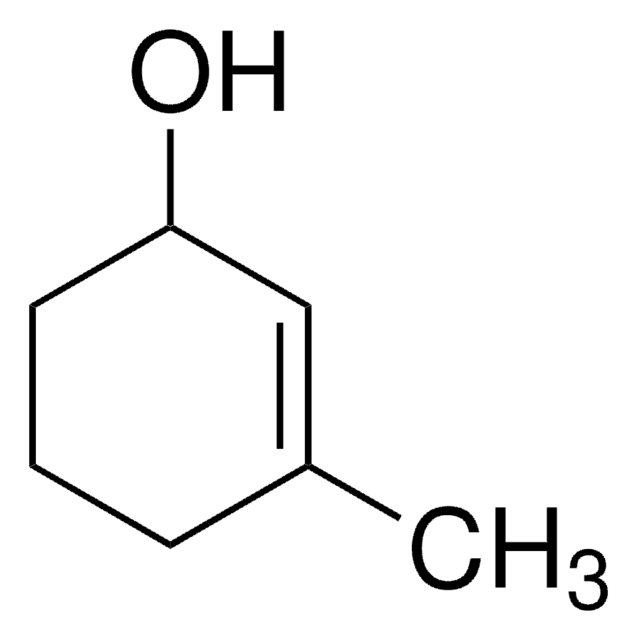
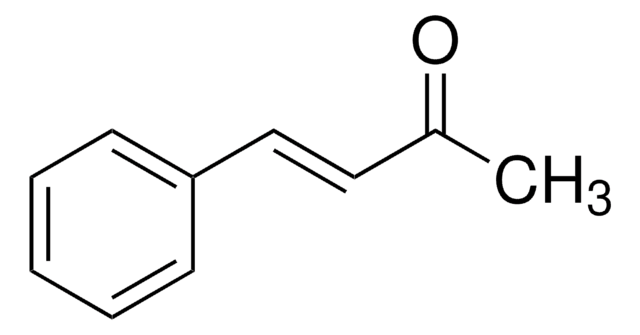
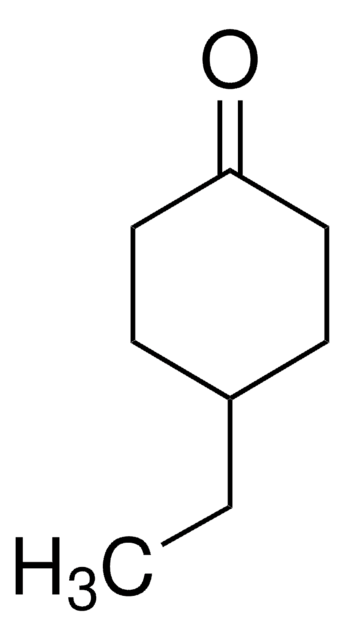
Linear Formula:
CH3C6H7(=O)
CAS Number:
Molecular Weight:
110.15
Beilstein:
1560601
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.494 (lit.)
bp
199-200 °C (lit.)
density
0.971 g/mL at 25 °C (lit.)
SMILES string
CC1=CC(=O)CCC1
InChI
1S/C7H10O/c1-6-3-2-4-7(8)5-6/h5H,2-4H2,1H3
InChI key
IITQJMYAYSNIMI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Methyl-2-cyclohexenone is an insect sex pheromone of the Douglas-fir beetle. It can be used as a starting material:
- In the total synthesis of (−)-ar-tenuifolene, a naturally occurring aromatic sesquiterpene.
- To synthesize an organic building block 2-trimethylsilyl-3-methyl-cyclohexenone.
- In the total synthesis of natural diterpenoids (+)-taiwaniaquinone H and (+)-dichroanone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Catalytic asymmetric formal total synthesis of (+)-dichroanone and (+)-taiwaniaquinone H
Li L-Q, et al.
Tetrahedron Letters, 55(43), 5960-5962 (2014)
Catalytic enantioselective total synthesis of (−)-ar-Tenuifolene
Shaw K, et al.
Tetrahedron Letters, 61(20), 151850-151850 (2020)
Aldrichimica Acta, 16, 41-41 (1983)
Shoichiro Horita et al.
Chembiochem : a European journal of chemical biology, 16(3), 440-445 (2015-02-03)
(4R,6R)-Actinol can be stereo-selectively synthesized from ketoisophorone by a two-step conversion using a mixture of two enzymes: Candida macedoniensis old yellow enzyme (CmOYE) and Corynebacterium aquaticum (6R)-levodione reductase. However, (4S)-phorenol, an intermediate, accumulates because of the limited substrate range of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service