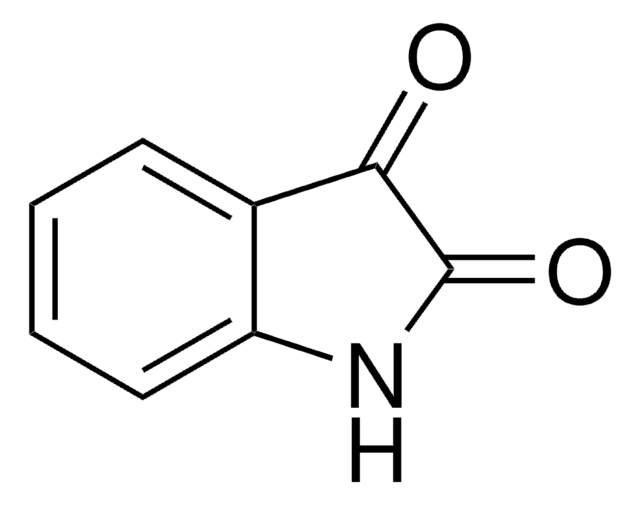
I12808
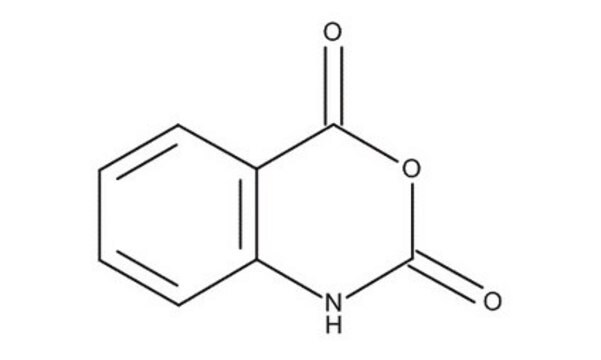
Isatoic anhydride
96%
Synonym(s):
3,1-Benzoxazine-2,4(1H)-dione, Anthranilic acid N-carboxylic acid anhydride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H5NO3
CAS Number:
Molecular Weight:
163.13
Beilstein:
136786
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
5.6 (vs air)
Assay
96%
mp
233 °C (dec.) (lit.)
SMILES string
O=C1Nc2ccccc2C(=O)O1
InChI
1S/C8H5NO3/c10-7-5-3-1-2-4-6(5)9-8(11)12-7/h1-4H,(H,9,11)
InChI key
TXJUTRJFNRYTHH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
586.4 °F - closed cup
Flash Point(C)
308 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jonathan J Goodall et al.
Chembiochem : a European journal of chemical biology, 3(1), 68-75 (2007-06-27)
The acyl-enzyme formed upon acylation of alpha-chymotrypsin with isatoic anhydride has been characterised by infrared spectroscopy. Acylation at pH 7 to yield the 2-aminobenzoyl-enzyme is rapid (k = 5.57x 10(-2)s(-1)), while deacylation is much slower (k =3.7 x 10(-5)10(-2) (s-).
P S Gravett et al.
The International journal of biochemistry, 23(10), 1101-1110 (1991-01-01)
1. Esterase E-I from Bitis gabonica was inactivated with irreversible inhibitors which included studies with a water-soluble carbodiimide, an affinity labelling peptide and a mechanism-based inactivator. 2. The reaction with 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide was biphasic and the dominant part followed saturation kinetics.
Zheng-Hui Guan et al.
Journal of the American Chemical Society, 134(42), 17490-17493 (2012-10-12)
A Pd-catalyzed regioselective C-H bond carbonylation of N-alkyl anilines for the synthesis of isatoic anhydrides has been developed. The key Pd-catalyst intermediate has been isolated and characterized. This novel Pd-catalyzed carbonylation reaction tolerates a wide range of functional groups and
K Siva Kumar et al.
Organic & biomolecular chemistry, 10(15), 3098-3103 (2012-03-10)
A one-pot cascade reaction has been developed leading to the concurrent construction of six and five membered fused N-heterocyclic rings of indazolo[3,2-b]quinazolinones. The methodology involved the reaction of isatoic anhydride, a hydrazine and o-iodo benzaldehyde in the presence of Pd(PPh(3))(4)
M H Gelb et al.
Journal of medicinal chemistry, 29(4), 585-589 (1986-04-01)
Derivatives of isatoic anhydride were prepared and tested as inhibitors of serine proteases. A number of isatoic anhydrides with positively charged substituents irreversibly inactivated several trypsin-like enzymes and preferentially inactivated trypsin over chymotrypsin. Further selectivity was obtained by introduction of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service