H20008
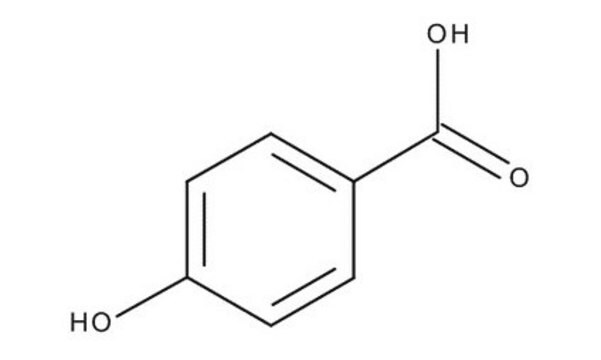
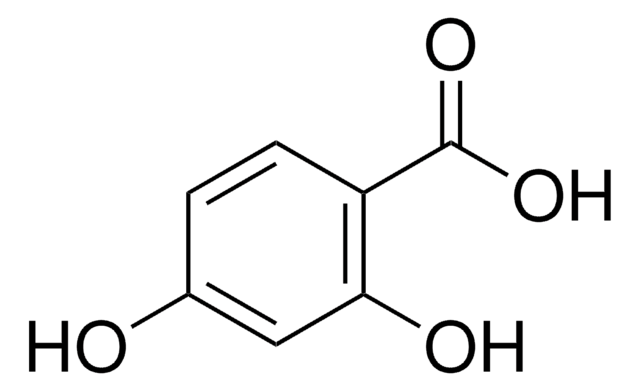
3-Hydroxybenzoic acid
ReagentPlus®, 99%
Synonym(s):
m-Salicylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4CO2H
CAS Number:
Molecular Weight:
138.12
Beilstein:
508160
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
mp
200-203 °C (lit.)
SMILES string
OC(=O)c1cccc(O)c1
InChI
1S/C7H6O3/c8-6-3-1-2-5(4-6)7(9)10/h1-4,8H,(H,9,10)
InChI key
IJFXRHURBJZNAO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Hydroxybenzoic acid, being a component of structural skeleton of various biologically active small molecules, is used in the synthesis of medicinally important compounds such as tyrosine kinase Tie-2 inhibitor, amyloidogenesis inhibitor, antioxidant and anti-inflammatory agents based on oxadiazole analogs of resveratrol.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Biochemical and structural evaluation of highly selective 2-arylbenzoxazole-based transthyretin amyloidogenesis inhibitors.
Johnson S M, et al.
Journal of Medicinal Chemistry, 51(2), 260-270 (2007)
Antioxidant and anti-inflammatory properties of 1, 2, 4-oxadiazole analogs of resveratrol.
Gobec M, et al.
Chemico-Biological Interactions, 240, 200-207 (2015)
Evolution of a highly selective and potent 2-(pyridin-2-yl)-1, 3, 5-triazine Tie-2 kinase inhibitor.
Hodous B L, et al.
Journal of Medicinal Chemistry, 50(4), 611-626 (2007)
Stefania Montersino et al.
Biochimica et biophysica acta, 1824(3), 433-442 (2011-12-31)
The genome of Rhodococcus jostii RHA1 contains an unusually large number of oxygenase encoding genes. Many of these genes have yet an unknown function, implying that a notable part of the biochemical and catabolic biodiversity of this Gram-positive soil actinomycete
Xianli Wu et al.
Molecular nutrition & food research, 53 Suppl 1, S76-S84 (2009-02-10)
Black raspberries (BRB) contain high levels of polyphenols and have been demonstrated to be chemopreventive. In order to investigate the underlying mechanism and study the metabolism of anthocyanins, pigs were fed freeze-dried BRB powder or purified diet (control) and three
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service