All Photos(1)
About This Item
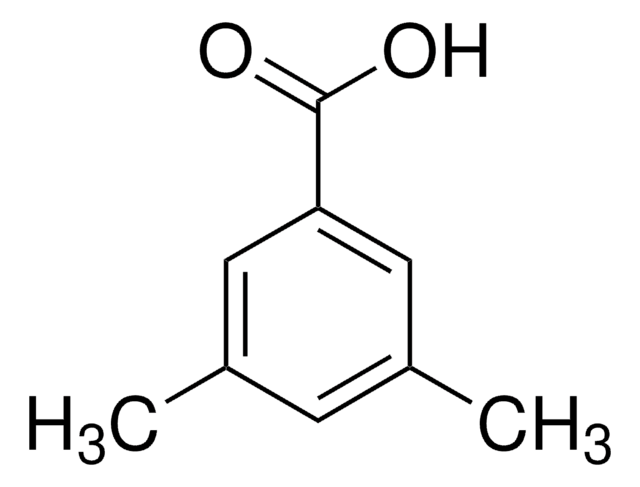
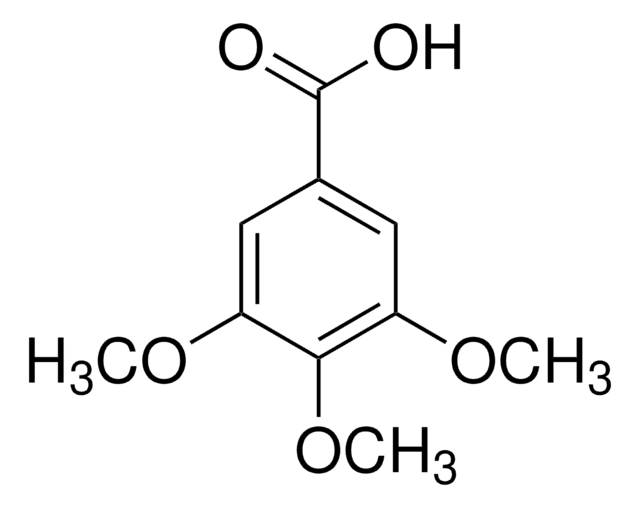
Linear Formula:
(CH3O)2C6H3CO2H
CAS Number:
Molecular Weight:
182.17
Beilstein:
511834
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
178-180 °C (lit.)
SMILES string
COc1cc(OC)cc(c1)C(O)=O
InChI
1S/C9H10O4/c1-12-7-3-6(9(10)11)4-8(5-7)13-2/h3-5H,1-2H3,(H,10,11)
InChI key
IWPZKOJSYQZABD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,5-Dimethoxybenzoic acid (3,5-DmeoxBA) can be used as a reactant for the synthesis of:
It can also be used as a ligand to synthesize lanthanide complexes [Ln(3,5-DmeoxBA)3(phen)]2; where phen is 1,10-phenanthroline.
- 5,7-Dimethoxy-3,4-diphenylisocoumarin by coupling with diphenylacetylene.
- Biotin dimedone, a reagent used in the study of protein sulfenation.
It can also be used as a ligand to synthesize lanthanide complexes [Ln(3,5-DmeoxBA)3(phen)]2; where phen is 1,10-phenanthroline.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rhodium-and iridium-catalyzed oxidative coupling of benzoic acids with alkynes via regioselective C- H bond cleavage.
Ueura K, et al.
The Journal of Organic Chemistry, 72(14), 5362-5367 (2007)
Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue.
Charles RL, et al.
Molecular and Cellular Proteomics, 6(9), 1473-1484 (2007)
Crystal structures, luminescence, and thermodynamic properties of lanthanide complexes with 3, 5-dimethoxybenzoic acid and 1, 10-phenanthroline.
Zheng JR, et al.
The Journal of Chemical Thermodynamics, 57(9), 169-177 (2013)
Alla V Lipeeva et al.
European journal of medicinal chemistry, 100, 119-128 (2015-06-17)
A series of 2-(4-R-triazolyl)substituted 3-oxo-2,3-dihydrofurocoumarins have been synthesized by a regioselective cycloaddition of 2-azidooreoselone 1 or 2-azido-9-[(4-methylpiperazin-1-yl)methyl]oreoselone 2 with various alkynes in the presence of Cu(II)/ascorbate in water/methylene chloride reaction medium. The structure of 2-azidooreoselone was established by X-ray structure
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service