B34680
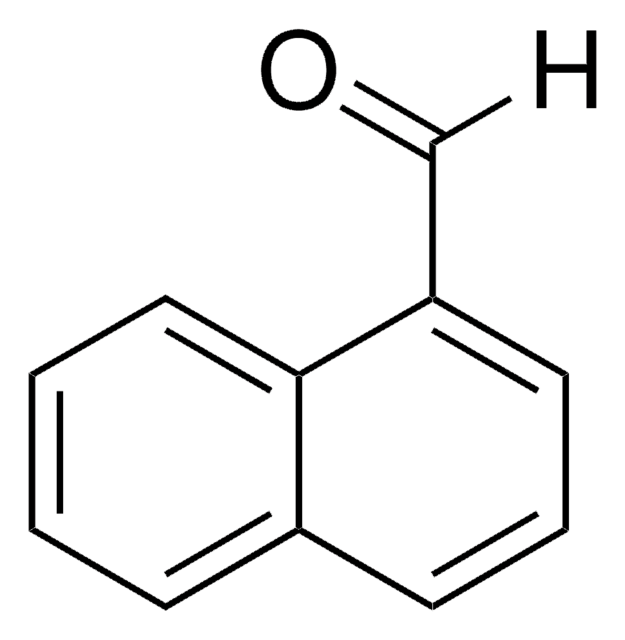
Biphenyl-4-carboxaldehyde
99%
Synonym(s):
4-Phenylbenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C6H4CHO
CAS Number:
Molecular Weight:
182.22
Beilstein:
606693
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
bp
184 °C/11 mmHg (lit.)
mp
57-59 °C (lit.)
SMILES string
O=Cc1ccc(cc1)-c2ccccc2
InChI
1S/C13H10O/c14-10-11-6-8-13(9-7-11)12-4-2-1-3-5-12/h1-10H
InChI key
ISDBWOPVZKNQDW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
352.0 - 381.9 °F
Flash Point(C)
177.8 - 194.4 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yuki Okabe et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 22(5), 713-725 (2017-01-14)
The construction of molecular catalysts that are active toward CO
Danuta Sek et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 175, 24-35 (2016-12-25)
The new Schiff bases bearing anthracene unit were synthesized from 2-aminoanthracene and various aldehydes such as: benzaldehyde, 4-(diphenylamino)benzaldehyde, 9-phenanthrenecarboxaldehyde, 9-anthracenecarboxaldehyde, and biphenyl-4-carboxaldehyde, 2-naphthaldehyde. Resulted azomethines were characterized by IR, NMR (
Sorel Jatunov et al.
Carbohydrate polymers, 123, 288-296 (2015-04-07)
A variety of fluorescent imino and secondary amino chitosans were synthesized under very mild conditions by reaction of the biopolymer amino functions with aromatic aldehydes in an acidified methanolic suspension. Simultaneous reactions of several aldehydes with chitosan were successfully carried
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![4-[N-(2,4-Diamino-6-pteridinylmethyl)-N-methylamino]benzoic acid hemihydrochloride hydrate 95%](/deepweb/assets/sigmaaldrich/product/structures/322/449/9a8d4b73-d9c4-4692-93d4-16a7a81d35c4/640/9a8d4b73-d9c4-4692-93d4-16a7a81d35c4.png)