All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C5H10O5
CAS Number:
Molecular Weight:
150.13
Beilstein:
1723085
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
optical activity
[α]20/D +103°, c = 1 in H2O
mp
160-163 °C (lit.)
SMILES string
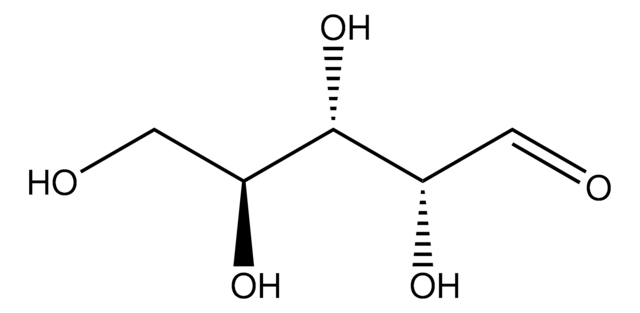
OC[C@H](O)[C@H](O)[C@@H](O)C=O
InChI
1S/C5H10O5/c6-1-3(8)5(10)4(9)2-7/h1,3-5,7-10H,2H2/t3-,4-,5+/m0/s1
InChI key
PYMYPHUHKUWMLA-VAYJURFESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
L-(+)-Arabinose is used as a key starting material in the total synthesis of (+)-ambruticin, zaragozic acid A, (−)-radicamine B and (+)-herbarumin I.
Biochem/physiol Actions
L-Arabinose is the naturally occurring isomer and is a constituent of plant polysaccharides. Most bacteria contain an inducible arabinose operon that codes for a series of enzymes and transporters that allows L-arabinose to be used as the sole carbon source in microbial culture.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Total synthesis of (+)-herbarumin I via intermolecular Nozaki-Hiyama-Kishi reaction.
Sabino, Adao Aparecido and Pilli, Ronaldo A
Tetrahedron Letters, 43(15), 2819-2821 (2002)
Total synthesis of (-)-radicamine B.
Gurjar, Mukund K et al.
Tetrahedron Letters, 47(39), 6979-6981 (2006)
Stereoselective total synthesis of zaragozic acid A based on an acetal [1, 2] Wittig rearrangement.
Tomooka, Katsuhiko et al.
Angewandte Chemie (International Edition in English), 39(24), 4502-4505 (2000)
Catarina M S S Neves et al.
Frontiers in chemistry, 7, 459-459 (2019-07-19)
The food industry produces significant amounts of waste, many of them rich in valuable compounds that could be recovered and reused in the framework of circular economy. The development of sustainable and cost-effective technologies to recover these value added compounds
Total synthesis of ambruticin.
Eun Lee et al.
Angewandte Chemie (International ed. in English), 41(1), 176-178 (2002-12-20)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service