All Photos(1)
About This Item
Empirical Formula (Hill Notation):
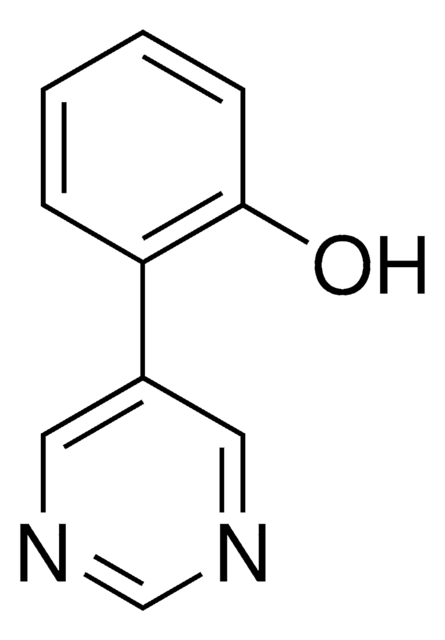
C11H9N2O
Molecular Weight:
185.20
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.06
Recommended Products
form
powder or chunks
Quality Level
mp
119-120 °C
functional group
aldehyde
storage temp.
−20°C
SMILES string
n1cncc(c1)c2c(cccc2)C=O
InChI
1S/C11H8N2O/c14-7-9-3-1-2-4-11(9)10-5-12-8-13-6-10/h1-8H
Application
2-(Pyrimidin-5-yl)benzaldehyde is a temporary directing group (TDG) to assist as a co-catalyst for metal catalyzed C-H functionalization. Often in C-H functionalization, an auxiliary compound is used to control site selectivity. These traditionally are covalently bonded to the compound of interest, and must subsequently be removed after functionalization, like a typical protecting group. To simplify the process of C-H functionalization, 2-fluoro-6-(pyrimidin-5-yl)aniline is one of a series of temporary directing groups developed by Deb Maiti′s lab that promote site selectivity without the inclusion of additional synthetic steps.
2-(pyrimidin-5-yl)benzaldehyde is an effective TDG for meta directed C-H functionalization of amine substituted target compounds, with high selectivity.
2-(pyrimidin-5-yl)benzaldehyde is an effective TDG for meta directed C-H functionalization of amine substituted target compounds, with high selectivity.
Other Notes
Imine as a linchpin approach for meta-C–H functionalization
https://www.nature.com/articles/s41570-021-00311-3">Transient directing ligands for selective metal-catalysed C–H activation
https://www.nature.com/articles/s41570-021-00311-3">Transient directing ligands for selective metal-catalysed C–H activation
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Transient directing ligands for selective metal-catalysed C?H activation
Nupur, et al.
Nature Reviews Chemistry, 5, 646?659-646?659 (2021)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/809/974/e027b628-7c2e-4bde-be7e-f9298d0c8b04/640/e027b628-7c2e-4bde-be7e-f9298d0c8b04.png)
