858781
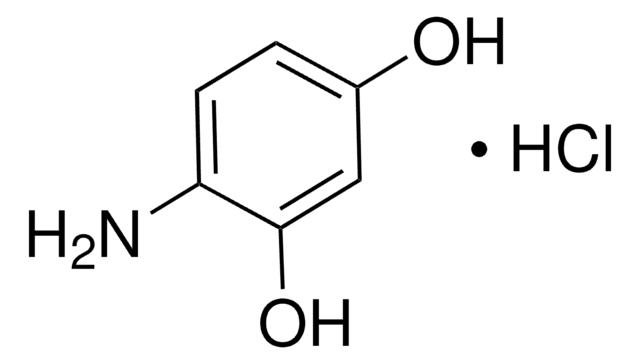
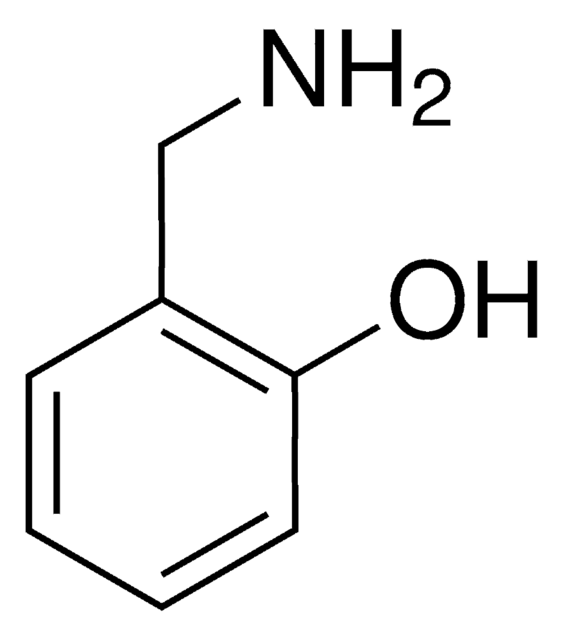
3,4-Dihydroxybenzylamine hydrobromide
98%
Synonym(s):
4-(Aminomethyl)catechol hydrobromide, DHBA hydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)2C6H3CH2NH2 · HBr
CAS Number:
Molecular Weight:
220.06
Beilstein:
4002646
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
184-186 °C (lit.)
SMILES string
Br.NCc1ccc(O)c(O)c1
InChI
1S/C7H9NO2.BrH/c8-4-5-1-2-6(9)7(10)3-5;/h1-3,9-10H,4,8H2;1H
InChI key
BVFZTXFCZAXSHN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
<ul>
<li><strong>Oxidative Polymerization of 3,4-Dihydroxybenzylamine:</strong> 3,4-Dihydroxybenzylamine is used in the synthesis of poly[3,4-dihydroxybenzylamine] (PDHBA) by oxidative polymerization, exploring its application as a lower homolog of dopamine for potential use in synthetic pathways and materials science (Petran et al., 2023).</li>
<li><strong>Detection of Urinary Free Metanephrines:</strong> Utilizing 3,4-Dihydroxybenzylamine hydrobromide as an internal standard, this research enhances the detection accuracy of urinary free metanephrines for diagnosing pheochromocytomas and paragangliomas, showcasing its importance in clinical diagnostic applications (Wang et al., 2020).</li>
<li><strong>Development of HPLC-ECD Method:</strong> A study developed an HPLC-ECD method using 3,4-Dihydroxybenzylamine as an internal standard for the analysis of vitamin C in plasma, demonstrating the chemical’s utility in enhancing analytical methodologies in biochemical research (Clark and Frank, 2016).</li>
<li><strong>Fluorescence Analysis of Catecholamines:</strong> 3,4-Dihydroxybenzylamine is used as an internal standard to determine catecholamines and related compounds in rat brain tissue, underlining its application in neurochemical analysis and research (Fonseca et al., 2017).</li>
</ul>
<li><strong>Oxidative Polymerization of 3,4-Dihydroxybenzylamine:</strong> 3,4-Dihydroxybenzylamine is used in the synthesis of poly[3,4-dihydroxybenzylamine] (PDHBA) by oxidative polymerization, exploring its application as a lower homolog of dopamine for potential use in synthetic pathways and materials science (Petran et al., 2023).</li>
<li><strong>Detection of Urinary Free Metanephrines:</strong> Utilizing 3,4-Dihydroxybenzylamine hydrobromide as an internal standard, this research enhances the detection accuracy of urinary free metanephrines for diagnosing pheochromocytomas and paragangliomas, showcasing its importance in clinical diagnostic applications (Wang et al., 2020).</li>
<li><strong>Development of HPLC-ECD Method:</strong> A study developed an HPLC-ECD method using 3,4-Dihydroxybenzylamine as an internal standard for the analysis of vitamin C in plasma, demonstrating the chemical’s utility in enhancing analytical methodologies in biochemical research (Clark and Frank, 2016).</li>
<li><strong>Fluorescence Analysis of Catecholamines:</strong> 3,4-Dihydroxybenzylamine is used as an internal standard to determine catecholamines and related compounds in rat brain tissue, underlining its application in neurochemical analysis and research (Fonseca et al., 2017).</li>
</ul>
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Surtees et al.
Analytical biochemistry, 181(2), 331-335 (1989-09-01)
A method for the measurement of S-adenosylmethionine in cerebrospinal fluid and brain using high-performance liquid chromatography with electrochemical detection is described. The method is based upon the catechol O-methyltransferase-catalyzed methylation of dihydroxybenzylamine to its 3- and 4-methoxy derivatives and measurement
G Santagostino et al.
Farmaco (Societa chimica italiana : 1989), 46(10), 1217-1223 (1991-10-01)
A simple routine method is described for simultaneous assay of total urinary norepinephrine, epinephrine, dopamine, normetanephrine and metanephrine. An internal standard of 3,4 dihydroxybenzylamine is added to the diluted urine and acidic hydrolysis of the conjugates is followed by reverse-phase
C Gerin et al.
Journal of neuroscience methods, 66(2), 81-92 (1996-06-01)
The aim of the microdialysis technique is to reflect as closely as possible the status and fluctuations of substances contained in the extracellular space. Most often, microdialysis is performed with repetitively implanted probes. We have recently devised an experimental set-up
M Sugumaran
Pigment cell research, 8(5), 250-254 (1995-10-01)
Dopamine and related compounds are known to be toxic to melanoma cells. Some of their toxicity may be related, in part, to the oxidation products generated from them upon their interaction with melanogenic enzymes. In this paper, we present our
Y Ikarashi et al.
Journal of chromatography. A, 718(2), 267-272 (1995-12-22)
A novel carbon material, plastic formed carbon (PFC), was prepared by mixing various amounts of pure graphite with an organic binder and pyrolysing the mixture to a "glassy carbon" at a modest final temperature of 1000-1400 degrees C. This preparation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service