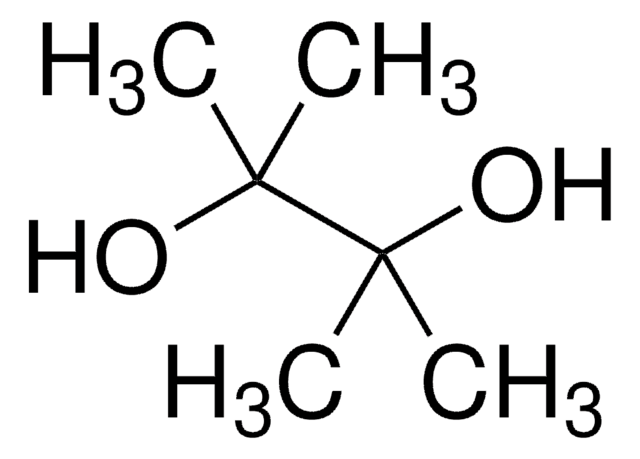
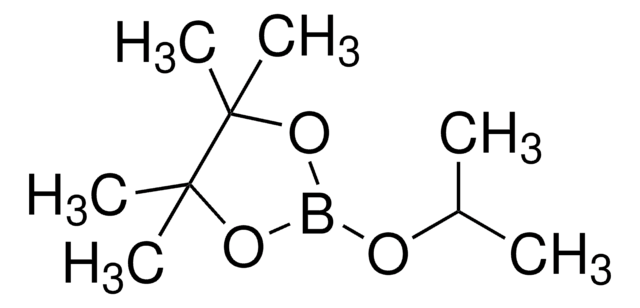
655856
4,4,5,5-Tetramethyl-1,3,2-dioxaborolane
97%
Synonym(s):
HBpin, Pinacolborane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H13BO2
CAS Number:
Molecular Weight:
127.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.396 (lit.)
bp
42-43 °C/50 mmHg (lit.)
density
0.882 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC1(C)OBOC1(C)C
InChI
1S/C6H13BO2/c1-5(2)6(3,4)9-7-8-5/h7H,1-4H3
InChI key
UCFSYHMCKWNKAH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4,4,5,5-Tetramethyl-1,3,2-dioxaborolane may be used for:
- Borylation at the benzylic C-H bond of alkylbenzenes in the presence of a palladium catalyst to form pinacol benzyl boronate.
- Hydroboration of alkyl or aryl alkynes and alkenes in the presence of transition metal catalysts.
- Coupling with aryl iodides in the presence of a copper catalyst to form aryl boronates.
- Asymmetric hydroboration of 1,3-enynes to form chiral allenyl boronates.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Water-react 2
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
41.0 °F - closed cup
Flash Point(C)
5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Copper-Catalyzed Asymmetric Hydroboration of 1, 3-Enynes with Pinacolborane to Access Chiral Allenylboronates.
Sang HL
Organic Chemistry Frontiers : An International Journal of Organic Chemistry / Royal Society of Chemistry (2018)
A study of hydroboration of alkenes and alkynes with pinacolborane catalyzed by transition metals.
Pereira S
Tetrahedron Letters, 37(19), 3283-3286 (1996)
Formation of arylboronates by a CuI-catalyzed coupling reaction of pinacolborane with aryl iodides at room temperature.
Zhu W
Organic Letters, 8(2), 261-263 (2006)
Palladium-catalyzed benzylic C?H borylation of alkylbenzenes with bis (pinacolato) diboron or pinacolborane.
Ishiyama T
Chemistry Letters (Jpn), 30(11), 1082-1083 (2001)
Articles
Ir(I)-Catalyzed C–H Borylation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![Bis[(pinacolato)boryl]methane](/deepweb/assets/sigmaaldrich/product/structures/286/283/dcb13110-c536-4223-99e6-0dd505906b64/640/dcb13110-c536-4223-99e6-0dd505906b64.png)