594806
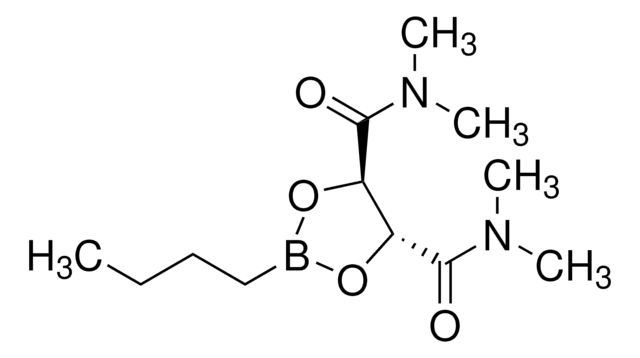
Butylboronic acid N,N,N′,N′-tetramethyl-D-tartaric acid diamide ester
97%
Synonym(s):
(4S,5S)-2-Butyl-N,N,N′N′-tetramethyl-1,3,2-dioxaborolane-4,5-dicarboxamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H23BN2O4
CAS Number:
Molecular Weight:
270.13
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
optical activity
[α]20/D +106°, c = 1% in chloroform
refractive index
n20/D 1.4780 (lit.)
bp
305-306 °C (lit.)
density
1.096 g/mL at 25 °C (lit.)
functional group
amide
SMILES string
CCCCB1O[C@@H]([C@H](O1)C(=O)N(C)C)C(=O)N(C)C
InChI
1S/C12H23BN2O4/c1-6-7-8-13-18-9(11(16)14(2)3)10(19-13)12(17)15(4)5/h9-10H,6-8H2,1-5H3/t9-,10-/m0/s1
InChI key
AFQWQRBBIZKYTE-UWVGGRQHSA-N
Related Categories
Application
Butylboronic acid N,N,N′,N′-tetramethyl-D-tartaric acid diamide is a dioxaborolane ligand that can be used to prepare:
- Enantioenriched spiropentanes via zinc catalyzed cyclopropanation of corresponding hydroxymethylallenes.
- 1,2,3-trisubstituted cyclopropane derivatives by asymmetric cyclopropanation of allylic alcohols in the presence of zinc reagents.
- Glycolipids plakosides A, B, and their derivatives by Sharpless asymmetric dihydroxylation and cyclopropanation reaction.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enantioselective synthesis of 1, 2, 3-trisubstituted cyclopropanes using gem-dizinc reagents
Zimmer LE and Charette AB
Journal of the American Chemical Society, 131(43), 15624-15626 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service