554073
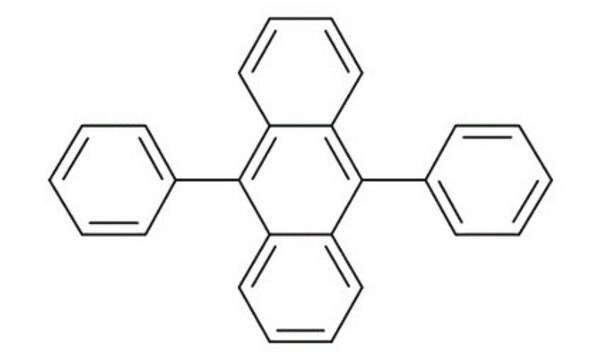
Rubrene
≥98%
Synonym(s):
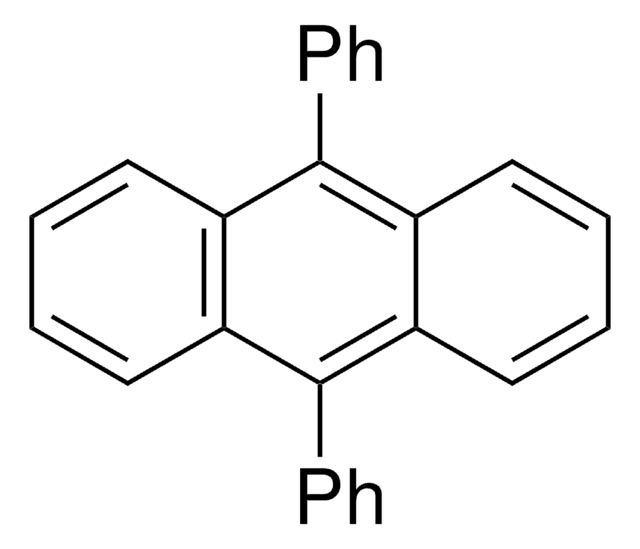
5,6,11,12-Tetraphenylnaphthacene
About This Item
Recommended Products
Quality Level
Assay
≥98%
mp
330-335 °C (lit.)
λmax
299 nm
460 nm (2nd)
SMILES string
c1ccc(cc1)-c2c3ccccc3c(-c4ccccc4)c5c(-c6ccccc6)c7ccccc7c(-c8ccccc8)c25
InChI
1S/C42H28/c1-5-17-29(18-6-1)37-33-25-13-14-26-34(33)39(31-21-9-3-10-22-31)42-40(32-23-11-4-12-24-32)36-28-16-15-27-35(36)38(41(37)42)30-19-7-2-8-20-30/h1-28H
InChI key
YYMBJDOZVAITBP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Organic Semiconductor Laser Materials
Graphene has emerged as the new wonder material. Being only one atom thick and composed of carbon atoms arranged in a hexagonal honeycomb lattice structure, the interest in this material has exploded exponentially since 2004 when it was first isolated and identified using a very simple method.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service