All Photos(1)
About This Item
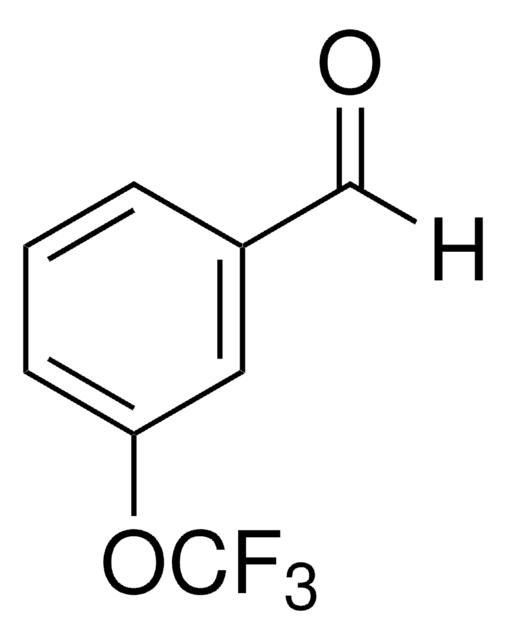
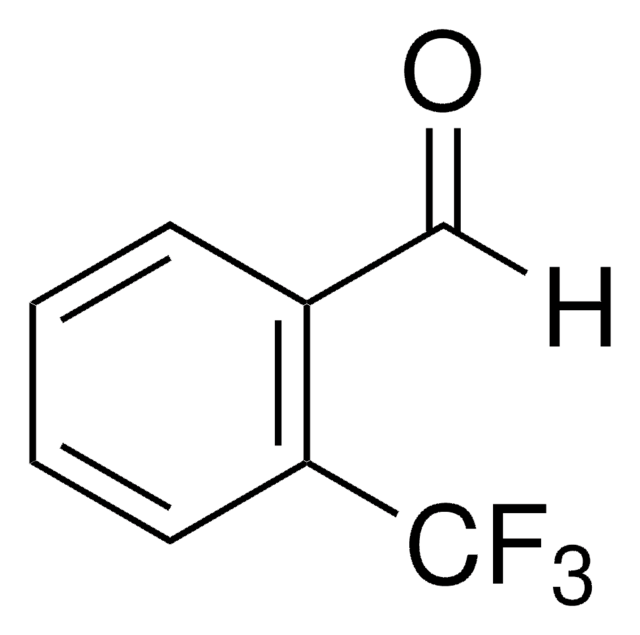
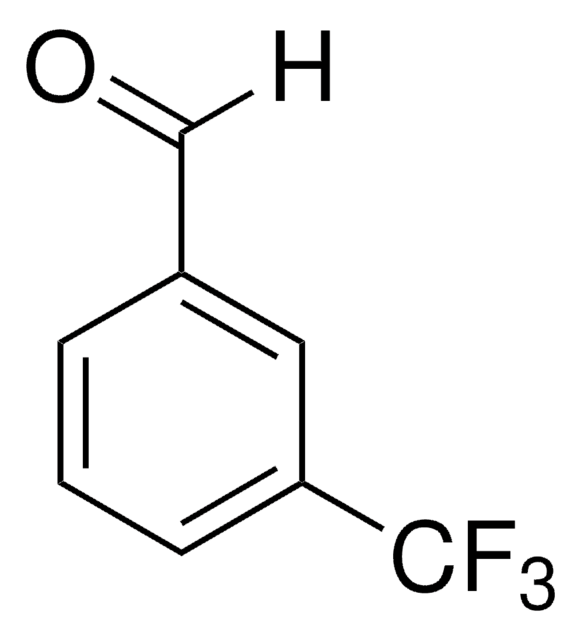
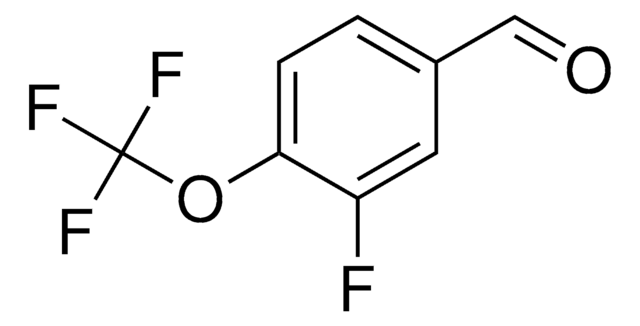
Linear Formula:
F3COC6H4CHO
CAS Number:
Molecular Weight:
190.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.454 (lit.)
bp
77 °C/20 mmHg (lit.)
density
1.332 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)Oc1ccccc1C=O
InChI
1S/C8H5F3O2/c9-8(10,11)13-7-4-2-1-3-6(7)5-12/h1-5H
InChI key
CPHXLFKIUVVIOQ-UHFFFAOYSA-N
Application
2-(Trifluoromethoxy)benzaldehyde may be used in the synthesis of tert-butyl N-[4-chloro-2-[α-hydroxy-α-2-(trifluoromethoxy)phenyl]]carbamate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
153.0 °F - closed cup
Flash Point(C)
67.2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Frédéric Leroux et al.
The Journal of organic chemistry, 68(12), 4693-4699 (2003-06-07)
Trifluoromethoxy-substituted anilines undergo hydrogen/lithium permutation ("metalation") with optional site selectivity depending on the N-protective group employed. N-tert-Butoxycarbonyl-2- and -4-(trifluoromethoxy)aniline react with tert-butyllithium at the nitrogen-adjacent 6- and 2-position affording, after electrophilic trapping, products 1-6. In contrast, deprotonation of the para
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service