All Photos(1)
About This Item
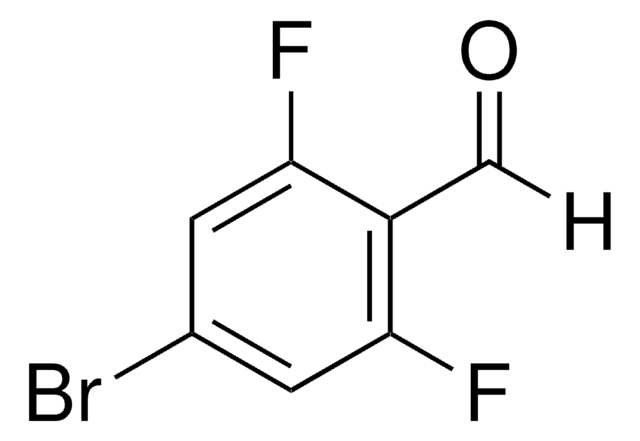
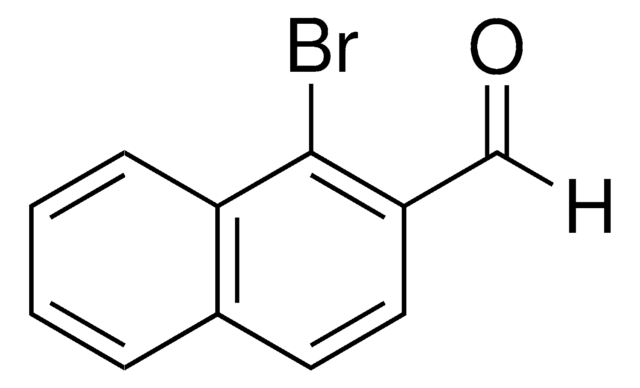
Linear Formula:
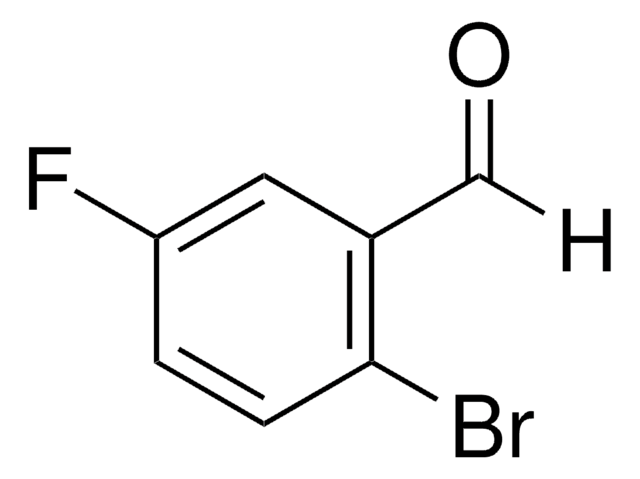
BrC6H3(F)CHO
CAS Number:
Molecular Weight:
203.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.57 (lit.)
bp
230 °C (lit.)
density
1.71 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
Fc1ccc(Br)cc1C=O
InChI
1S/C7H4BrFO/c8-6-1-2-7(9)5(3-6)4-10/h1-4H
InChI key
MMFGGDVQLQQQRX-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
228.9 °F - closed cup
Flash Point(C)
109.4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
High throughput synthesis of diverse 2, 5-disubstituted indoles using titanium carbenoids bearing boronate functionality.
Main CA, et al.
Tetrahedron, 64(5), 901-914 (2008)
Synthesis of 5-cyanoindazole and 1-methyl and 1-aryl-5-cyanoindazoles.
Halley F and Sava X.
Synthetic Communications, 27(7), 1199-1207 (1997)
Christian Ruzié et al.
The Journal of organic chemistry, 78(15), 7741-7748 (2013-07-16)
The synthesis of 1,6-, 2,7-, 3,8-, and 4,9-isomers of dibromo- and didodecyl[1]benzothieno[3,2-b][1]benzothiophenes, via the stilbene pathway, is described. Starting from the synthesis of bromo-2-(methylthio)benzaldehydes, a series of functionalization, McMurry coupling, and finalising cyclization reactions were explored. The stereochemistry of the
Keith W Woods et al.
Bioorganic & medicinal chemistry, 14(20), 6832-6846 (2006-07-18)
A series of heteroaryl-pyridine containing inhibitors of Akt are reported. The synthesis and structure-activity relationships are discussed, leading to the discovery of a indazole-pyridine analogue (K(i)=0.16 nM). These compounds bind in the ATP binding site, are potent, ATP competitive, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service