526169
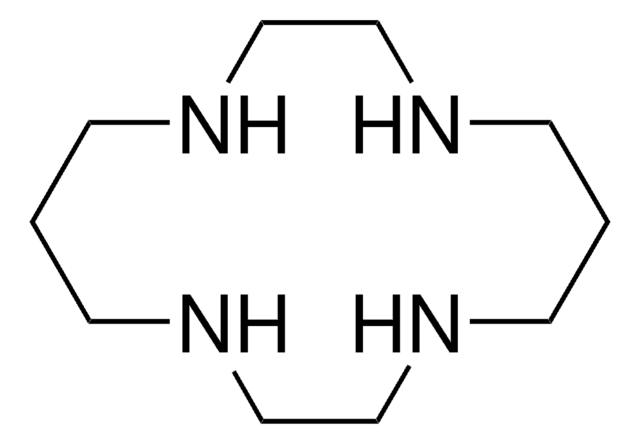
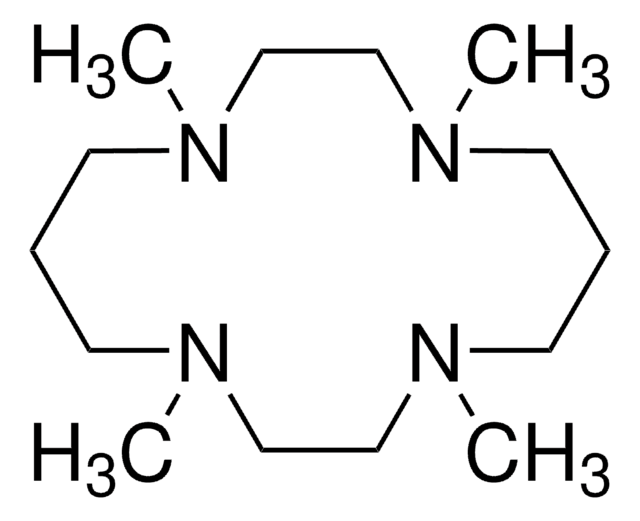
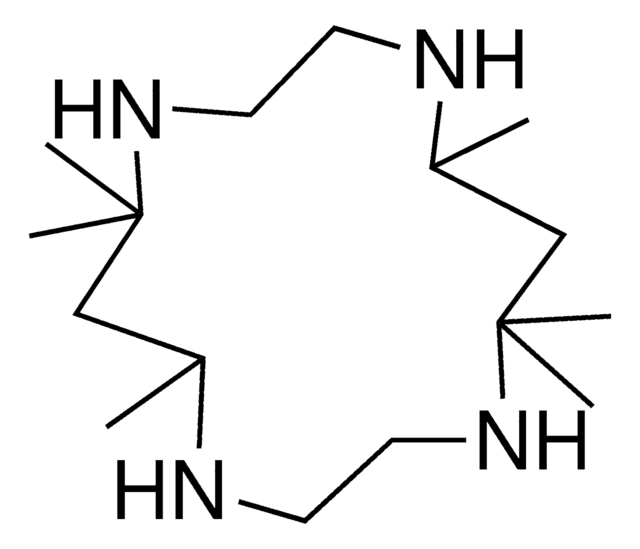
1,8-Dimethyl-1,4,8,11-tetraazacyclotetradecane
Synonym(s):
1,8-Dimethylcyclam
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H28N4
CAS Number:
Molecular Weight:
228.38
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.4940 (lit.)
bp
98-99 °C (lit.)
density
0.944 g/mL at 25 °C (lit.)
SMILES string
CN1CCCNCCN(C)CCCNCC1
InChI
1S/C12H28N4/c1-15-9-3-5-14-8-12-16(2)10-4-6-13-7-11-15/h13-14H,3-12H2,1-2H3
InChI key
BNLDMZVBFXARKJ-UHFFFAOYSA-N
Related Categories
General description
1,8-Dimethyl-1,4,8,11-tetraazacyclotetradecane is a heterocyclic building block.
Application
1,8-Dimethyl-1,4,8,11-tetraazacyclotetradecane may be used as a precursor for the synthesis of `trans′-difunctionalized derivatives. It may also be used in the synthesis of 4,11-bis(N-pyren-1-yl-acetamide)-1,8-dimethyl-1,4,8,11-tetraazacyclotetradecane.
Reactant for:
- Ferromagnetic coupling of copper(II) and nickel(II) complexes
- Preparation of cyclam bridged dinuclear platinum(II) complex as an antitumor agent
- Preparation of dimethylcyclam based fluoroionophore having Hg2+- and Cd2+-selective signaling behaviors
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
195.1 °F - closed cup
Flash Point(C)
90.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
So-Youn Moon et al.
The Journal of organic chemistry, 70(6), 2394-2397 (2005-03-12)
[reaction: see text] A new fluoroionophore has been synthesized by appending two signaling pyrenylacetamide subunits on the binding motif of 1,8-dimethylcyclam. The designed compound exhibited highly selective and sensitive fluoroionophoric behavior toward Hg(2+) ions of excimer emission in aqueous dioxane
Copper (II) and Nickel (II) Complexes oftrans'-Difunctionalized Tetraaza Macrocycles.
Comparone A and Kaden TA.
Helvetica Chimica Acta, 81(10), 1765-1772 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service