514381
Titanium(III) chloride
≥99.995% trace metals basis
Synonym(s):
Titanium trichloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
TiCl3
CAS Number:
Molecular Weight:
154.23
EC Number:
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
Recommended Products
Assay
≥99.995% trace metals basis
form
crystalline
reaction suitability
reagent type: catalyst
core: titanium
mp
440 °C (dec.) (lit.)
SMILES string
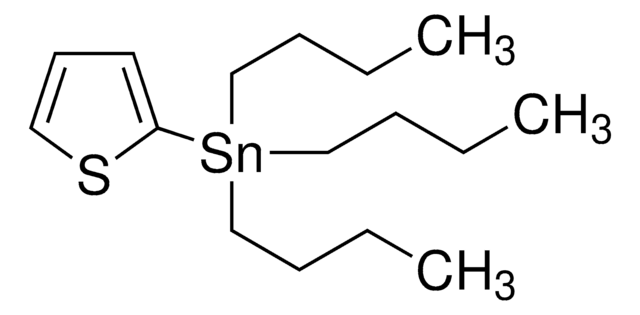
Cl[Ti](Cl)Cl
InChI
1S/3ClH.Ti/h3*1H;/q;;;+3/p-3
InChI key
YONPGGFAJWQGJC-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Application
Used for the vapor-phase formation of intermetallic compounds with ultrafine particle size.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Pyr. Sol. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gnana S Siluvai et al.
Biochemistry, 48(51), 12133-12144 (2009-11-20)
Sco-like proteins contain copper bound by two cysteines and a histidine residue. Although their function is still incompletely understood, there is a clear involvement with the assembly of cytochrome oxidases that contain the Cu(A) center in subunit 2, possibly mediating
Investigation into the effectiveness of surface treatment on poly(methyl methacrylate) when exposed in the mouth.
M D Murray
The Journal of prosthetic dentistry, 59(3), 368-373 (1988-03-01)
D J Kushner et al.
Analytical biochemistry, 133(1), 116-119 (1983-08-01)
Hydroxamic acid siderophores were observed to be inactivated by exposure to titanium(III) chloride. To study the reaction, a series of eight model hydroxamic acids were prepared and reacted with titanium(III) chloride. The products were shown by ir and NMR comparisons
Yan Zhang et al.
Organic & biomolecular chemistry, 9(13), 4977-4982 (2011-05-21)
N-N bond cleavage in hydrazines is widely used in the preparation of amines and thus occupies a significant place in organic synthesis. In this paper, we report a new method for the reductive cleavage of N-N bonds in hydrazines by
C P Offen et al.
Xenobiotica; the fate of foreign compounds in biological systems, 15(6), 503-511 (1985-06-01)
The metabolic fate of chlormethiazole in healthy male subjects has been investigated following the oral administration of a single dose of chlormethiazole edisylate. Five urinary C-oxidation metabolites were identified and shown to be identical with previously reported metabolites. A novel
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service