All Photos(1)
About This Item
Empirical Formula (Hill Notation):
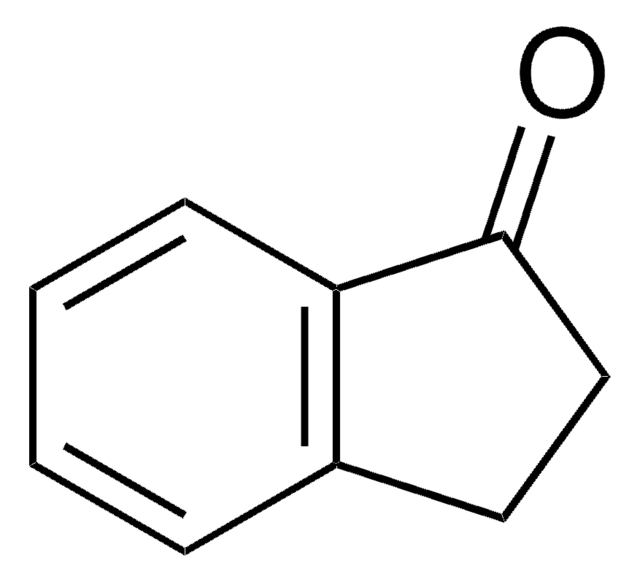
C9H8O2
CAS Number:
Molecular Weight:
148.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
impurities
<3% acetone
mp
175 °C (dec.) (lit.)
functional group
ketone
SMILES string
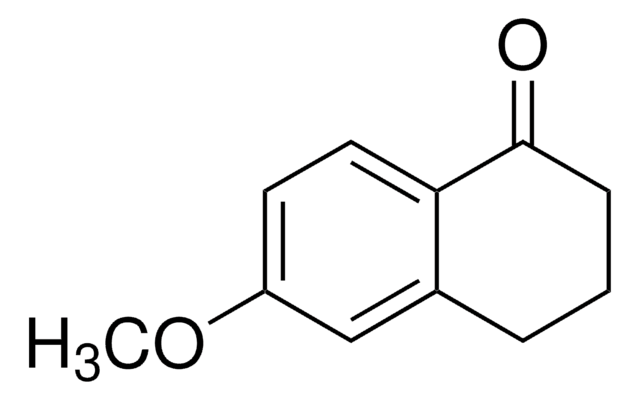
Oc1ccc2C(=O)CCc2c1
InChI
1S/C9H8O2/c10-7-2-3-8-6(5-7)1-4-9(8)11/h2-3,5,10H,1,4H2
InChI key
ZRKQOVXGDIZYDS-UHFFFAOYSA-N
General description
5-Hydroxy-1-indanone is a 1-indanone derivative.
Application
5-Hydroxy-1-indanone may be used in the preparation of (5-hydroxy-indan-(1E)-ylidene)-acetic acid and 5-[2-(phenyl)ethoxy]-1-indanone. It may be used as a starting material in the multi-step synthesis of 5H-indeno[1,2-c]pyridazin-5-one analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Geometric and solvent effects on intramolecular phenolic hydrogen abstraction by carbonyl n,p* and p,p* triplets.
Lathioor EC and Leigh WJ.
Canadian Journal of Chemistry, 79(12), 1851-1863 (2001)
On the involvement of single-bond rotation in the primary photochemistry of photoactive yellow protein.
Stahl AD, et al.
Biophysical Journal, 101(5), 1184-1192 (2011)
J Reniers et al.
European journal of medicinal chemistry, 46(12), 6104-6111 (2011-10-25)
Previous studies on 5H-indeno[1,2-c]pyridazin-5-one derivatives as inhibitors of MAO-B revealed that it was possible to increase the MAO-B inhibitory potency of 5H-indeno[1,2-c]pyridazin-5-ones by substituting the central heterocycle in the 3-position or C-8 with lipophilic groups which occupy the substrate cavity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service