493023
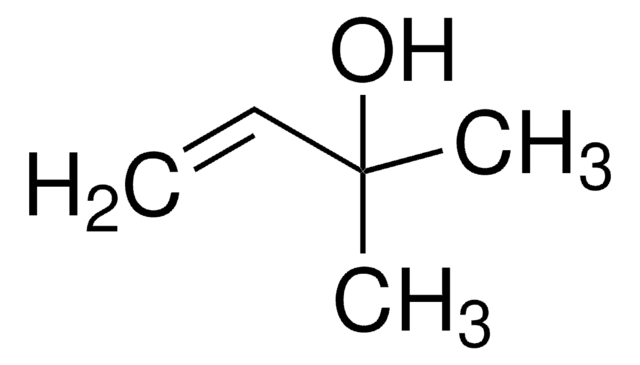
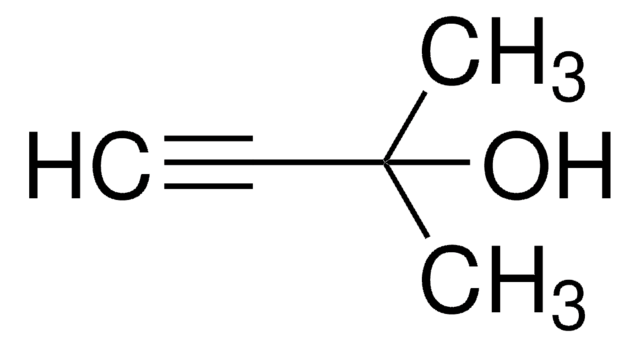
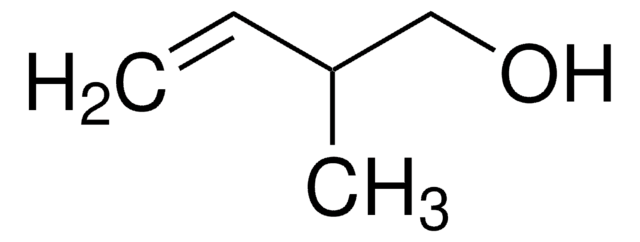
3-Methyl-1-penten-4-yn-3-ol
98%
Synonym(s):
Ethynyl methyl vinyl carbinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HC≡CC(CH3)(OH)CH=CH2
CAS Number:
Molecular Weight:
96.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.446 (lit.)
bp
63-65 °C/100 mmHg (lit.)
density
0.89 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CC(O)(C=C)C#C
InChI
1S/C6H8O/c1-4-6(3,7)5-2/h1,5,7H,2H2,3H3
InChI key
VBATUBQIYXCZPA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Methyl-1-penten-4-yn-3-ol is an acetylenic alcohol. It reacts with ruthenium vinyl carbene to form a ten-membered η2-olefin coordinated ruthenacycle. The efficiency of different palladium catalysts for the hydrogenation of 3-methyl-1-penten-4-yn-3-ol under continuous-flow liquid-phase conditions has been evaluated. It undergoes allylic rearrangement to form cis and trans isomers of 3-methyl-2-penten-4-yn-l-ol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
90.0 °F - closed cup
Flash Point(C)
32.2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Allyl rearrangement of 3-methyl-1-penten-4-yn-3-ol.
Skoda A, et al.
Chemical Papers, 43(6), 743-751 (1989)
1,2-Migration in the reactions of ruthenium vinyl carbene with propargyl alcohols.
Zhou X, et al.
Organic Chemistry Frontiers : An International Journal of Organic Chemistry / Royal Society of Chemistry, 1(9), 1077-1082 (2014)
From the Lindlar Catalyst to Supported Ligand-Modified Palladium Nanoparticles: Selectivity Patterns and Accessibility Constraints in the Continuous-Flow Three-Phase Hydrogenation of Acetylenic Compounds.
Vile G, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 20(20), 5926-5937 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service