420069
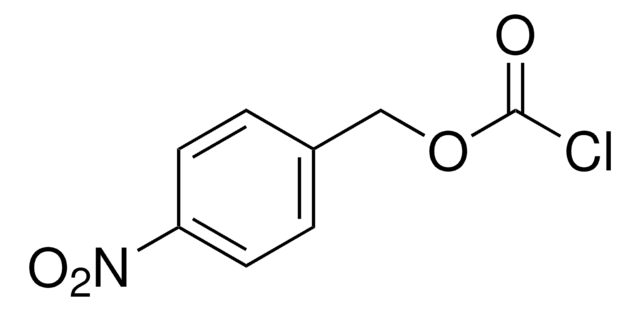
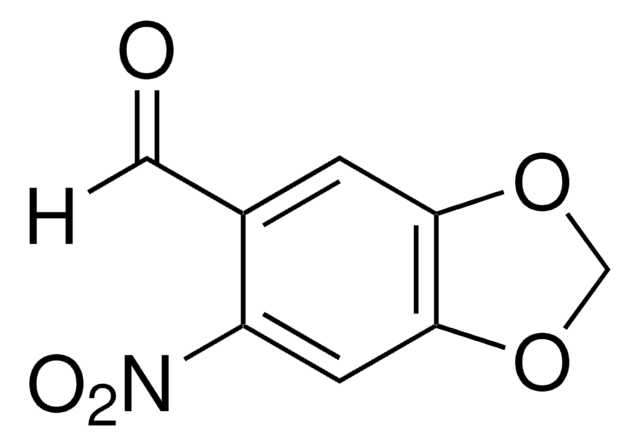
4,5-Dimethoxy-2-nitrobenzyl chloroformate
97%, for peptide synthesis
Synonym(s):
6-Nitroveratryl chloroformate, 6-Nitroveratryloxycarbonyl chloride, NVOC chloride, NVOC-Cl
About This Item
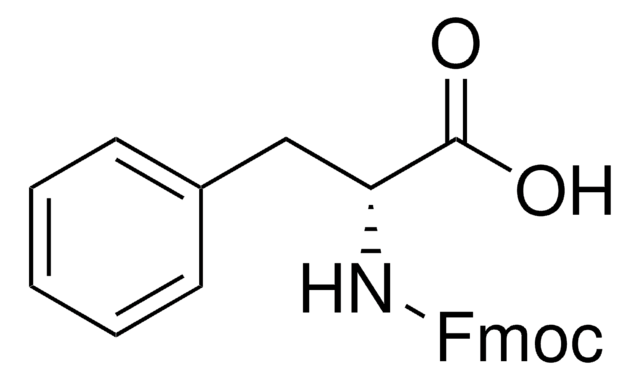
Recommended Products
product name
4,5-Dimethoxy-2-nitrobenzyl chloroformate, 97%
Quality Level
Assay
97%
form
powder
mp
125 °C (dec.) (lit.)
application(s)
peptide synthesis
functional group
chloro
nitro
storage temp.
2-8°C
SMILES string
COc1cc(COC(Cl)=O)c(cc1OC)[N+]([O-])=O
InChI
1S/C10H10ClNO6/c1-16-8-3-6(5-18-10(11)13)7(12(14)15)4-9(8)17-2/h3-4H,5H2,1-2H3
InChI key
RWWPKIOWBQFXEE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Preparation of inactive, caged protein conjugates which can be activated by irradiating near-ultraviolet light.
- Solid-phase synthesis of base-sensitive S-acylthioethyl (SATE)-prooligonucleotides.
- Modification of surface properties by introducing photocleavable NVOC moiety into chitosan to control cell attachment.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service