All Photos(1)
About This Item
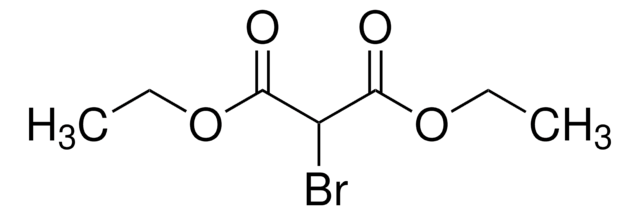
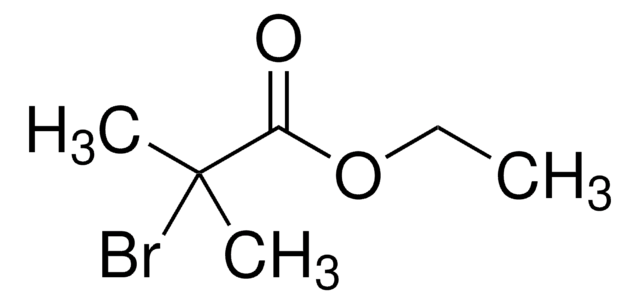
Linear Formula:
Br2C(CO2C2H5)2
CAS Number:
Molecular Weight:
317.96
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.484 (lit.)
bp
140-143 °C/18 mmHg (lit.)
density
1.68 g/mL at 25 °C (lit.)
functional group
bromo
ester
SMILES string
CCOC(=O)C(Br)(Br)C(=O)OCC
InChI
1S/C7H10Br2O4/c1-3-12-5(10)7(8,9)6(11)13-4-2/h3-4H2,1-2H3
InChI key
PFZYFZRUPFUEOB-UHFFFAOYSA-N
General description
Diethyl dibromomalonate reacts with sodium methoxide in cyclohexene to afford dibromonorcarane. It also reacts with allyl(pyridine)cobaloximes to afford the corresponding allyl-substituted esters.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A novel bromination for an unsaturated a-anion ester. Synthesis of 2-bromo-cis-8, cis-11, cis-14-eicosatrienoic acid.
van der Wolf L and Pabon HJJ.
Rec. Trav. Chim., 96(3), 72-74 (1977)
Reactions of organocobalt complexes with bromoesters: regiospecific synthesis of allyl-and cyclopropylmethyl-substituted malonic and acetoacetic esters.
Veber M, et al.
Journal of Organometallic Chemistry, 209(3), 393-399 (1981)
Dominik Schuch et al.
Journal of the American Chemical Society, 131(36), 12918-12920 (2009-08-22)
Tetrahydrofur-2-ylmethyl radicals were stereoselectively generated from substituted pent-4-en-1-ols in aerobic cobalt(II)-catalyzed oxidations. Intermediates were trapped with cyclohexa-1,4-diene, gamma-terpinene, BrCCl(3), diethyl dibromomalonate, or electron-deficient olefins such as acrylonitrile or dimethyl fumarate to afford functionalized tetrahydrofurans in synthetically useful yields.
Reaction of diethyl dibromomalonate with methoxide: Evidence for a novel bromophilic attack.
Mebane RC, et al.
Tetrahedron Letters, 40(8), 1459-1462 (1999)
Investigations into the Bromination of Substituted Phenols using Diethyl Bromomalonate and Diethyl Dibromomalonate.
Coumbarides GS, et al.
Bulletin of the Chemical Society of Japan, 74(1), 179-180 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service