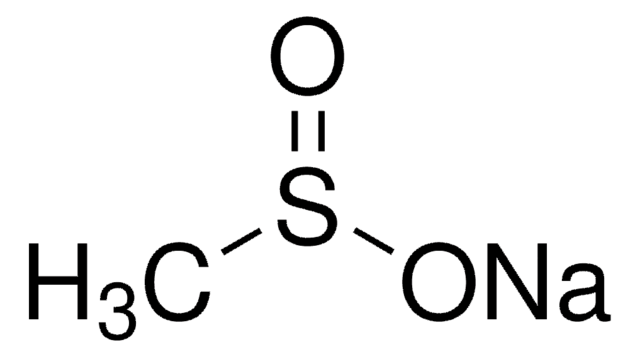
407240
Copper(I) trifluoromethanesulfonate benzene complex
technical grade, 90%
Synonym(s):
Copper(I) triflate benzene complex, Cuprous trifluoromethanesulfonate benzene complex, Trifluoromethanesulfonic acid copper(I) salt benzene complex
About This Item
Recommended Products
grade
technical grade
Assay
90%
form
powder
reaction suitability
core: copper
reagent type: catalyst
mp
160 °C (dec.) (lit.)
SMILES string
[Cu+].[Cu+].c1ccccc1.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/C6H6.2CHF3O3S.2Cu/c1-2-4-6-5-3-1;2*2-1(3,4)8(5,6)7;;/h1-6H;2*(H,5,6,7);;/q;;;2*+1/p-2
InChI key
GNXZWVVAAMVOJY-UHFFFAOYSA-L
Application
- To synthesize enol-esters via copper(I) carboxylate intermediate formation.
- In the enantioselective allylic oxidation of cyclic alkenes.
- To prepare 2,5-disubstituted pyrrolidine derivatives from N-alkenyl, alkynyl and alkyl N-benzoyloxysulfonamides via the sulfonamidyl radical formation.
It can also be used in combination with amino acid-based chiral phosphine ligands to catalyze asymmetric conjugate additions of alkylzincs to acyclic α,β-unsaturated ketones, affording β-alkylcarbonyls in high yield and with excellent enantioselectivity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service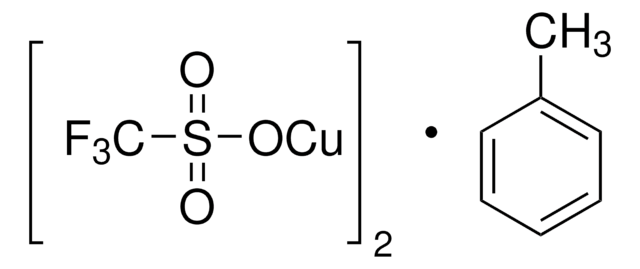

![Chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)