400424
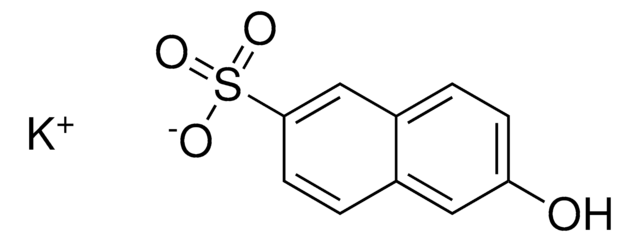
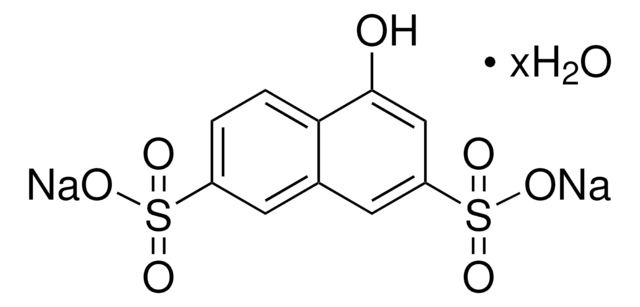
6-Hydroxy-2-naphthalenesulfonic acid sodium salt hydrate
technical grade
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC10H6SO3Na · xH2O
Molecular Weight:
246.21 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
solid
mp
>300 °C (lit.)
functional group
sulfonic acid
SMILES string
O.[Na+].Oc1ccc2cc(ccc2c1)S([O-])(=O)=O
InChI
1S/C10H8O4S.Na.H2O/c11-9-3-1-8-6-10(15(12,13)14)4-2-7(8)5-9;;/h1-6,11H,(H,12,13,14);;1H2/q;+1;/p-1
InChI key
QHVRFNCHOMKXQP-UHFFFAOYSA-M
General description
Sodium salt of 6-hydroxy-2-naphthalenesulfonic acid is also referred as Schaeffer′s salt.
Application
6-Hydroxy-2-naphthalenesulfonic acid sodium salt hydrate may be used in the following studies:
- As precursors during the agar-plate screening test.
- Preparation of methoxy-substituted naphthalenesulfonyl chloride.
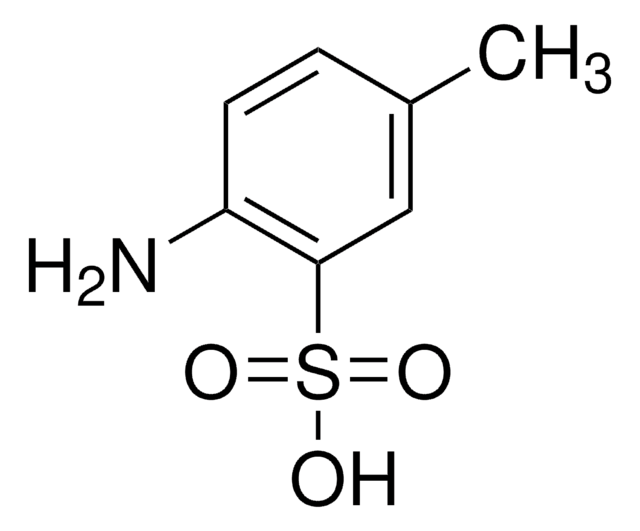
- Synthesis of FD&C Red No. 40, a synthetic water-soluble color that is permitted for coloring foods, drugs and cosmetics in the U.S.A.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anamarija Zega et al.
Bioorganic & medicinal chemistry letters, 14(6), 1563-1567 (2004-03-10)
A series of azaphenylalanine derivatives were investigated as novel thrombin inhibitors based on the prodrug principle. By systematic structural modifications we have identified optimal groups for this series that led us to potent inhibitors of thrombin incorporating the benzamidine fragment
N Richfield-Fratz et al.
Journal of chromatography, 467(1), 167-176 (1989-04-21)
The unsulfonated aromatic amine 4-nitro-p-cresidine (2-methoxy-5-methyl-4-nitrobenzenamine) was identified as an impurity in the regulated color additive FD&C Red. No. 40. The compound was isolated from the water-soluble color by extraction with chloroform, followed by transfer of the free amines to
Jolanta Polak et al.
Microbial cell factories, 9, 51-51 (2010-07-06)
Chemical methods of producing dyes involve extreme temperatures and unsafe toxic compounds. Application of oxidizing enzymes obtained from fungal species, for example laccase, is an alternative to chemical synthesis of dyes. Laccase can be replaced by fungal biomass acting as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service