359378
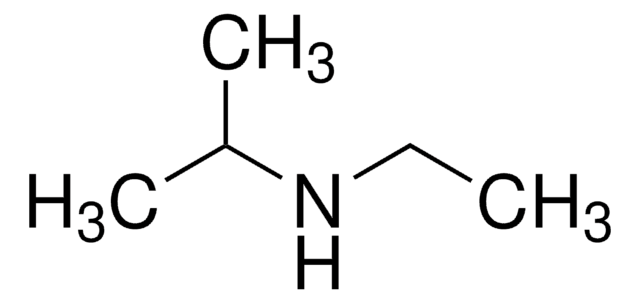
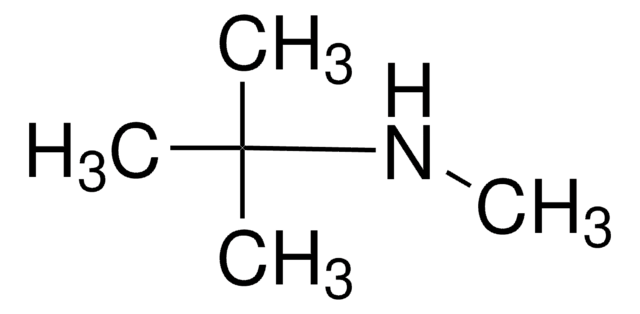
N-Isopropylmethylamine
98%
Synonym(s):
N-Methylisopropylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHNHCH3
CAS Number:
Molecular Weight:
73.14
Beilstein:
1730877
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
4.15 psi ( 20 °C)
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.384 (lit.)
bp
50-53 °C (lit.)
density
0.702 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CNC(C)C
InChI
1S/C4H11N/c1-4(2)5-3/h4-5H,1-3H3
InChI key
XHFGWHUWQXTGAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
N-Isopropylmethylamine is an unsymmetrical amine. Pd/C-catalyzed oxidative cross double carbonylation of N-isopropylmethylamine with alcohols has been reported.
Application
N-Isopropylmethylamine is suitable for use in the generation of in situ micelles having distinct sizes, by sequential self-assembly of a series of stimuli-responsive amphiphilic block copolymers, prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization. It is suitable for use in the synthesis of novel 5-ethoxy-N,N-dialkyl-[α,α,β,β-H4]-tryptamines and their [α,α,β,β,β−D4]-counterparts following the Speeter and Anthony procedure.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-25.6 °F - closed cup
Flash Point(C)
-32 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sandip T Gadge et al.
The Journal of organic chemistry, 78(13), 6793-6797 (2013-06-06)
This work reports a mild, efficient, and ligand-free Pd/C-catalyzed protocol for the oxidative cross double carbonylation of amines and alcohols. Notably, the reaction does not requires any base, co-catalyst, dehydrating agent, or ligand. Pd/C solves the problem of catalyst recovery
MULTIMODAL HIERARCHICAL ?FRACTAL-LIKE? MICELLAR SOLUTIONS OF THERMO-RESPONSIVE DOUBLE-HYDROPHILIC ACRYLAMIDE BLOCK COPOLYMERS SYNTHESIZED BY RAFT POLYMERIZATION.
Amado E, et al.
Polymer Preprints (American Chemical Society, Division of Polymer Chemistry), 52(2), 691-692 (2011)
Ruchanok Tearavarich et al.
Drug testing and analysis, 3(9), 597-608 (2011-10-01)
The increased interest in N,N-dialkyl tryptamines is a reflection of their diverse range of biologically active properties. Deuterated derivatives are of interest for use as internal standards in bioanalytical or pharmacological assays. The present study reports on the synthesis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service