All Photos(3)
About This Item
Linear Formula:
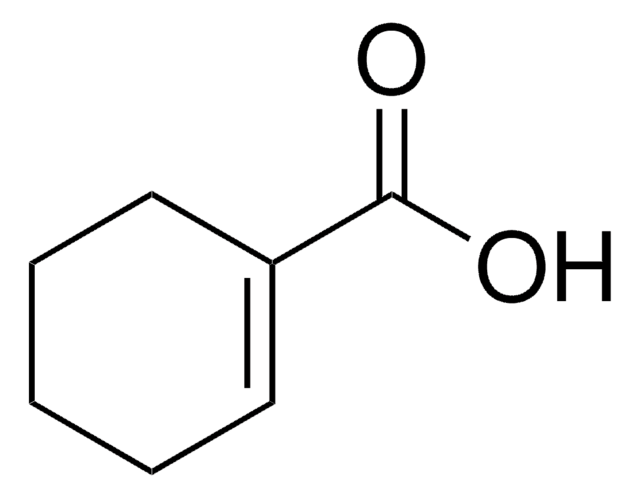
C5H7CO2H
CAS Number:
Molecular Weight:
112.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
121-124 °C (lit.)
SMILES string
OC(=O)C1=CCCC1
InChI
1S/C6H8O2/c7-6(8)5-3-1-2-4-5/h3H,1-2,4H2,(H,7,8)
InChI key
PYRZPBDTPRQYKG-UHFFFAOYSA-N
Related Categories
General description
1-Cyclopentenecarboxylic acid was evaluated as a new effective anticonvulsant during Anticonvulsant Screening Program (ASP) of Antiepileptic Drug Development Program.
Application
1-Cyclopentenecarboxylic acid was used in synthesis of cis-8-hexahydroindanecarboxylic acid via Diels-Alder reaction with butadiene. It was also used in synthesis of isoxazole derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Preparation of cis-Hexahydroindane-8-carboxylic Acid.
DAUBEN W, et al.
The Journal of Organic Chemistry, 26(2), 297-300 (1961)
Pengcheng P Shao et al.
Bioorganic & medicinal chemistry letters, 19(18), 5334-5338 (2009-08-18)
A series of novel isoxazole voltage gated sodium channel blockers have been synthesized and evaluated. Substitutions on the benzylic position of benzamide were investigated to determine their effect on Na(v)1.7 inhibitory potency. The spirocyclobutyl substitution had the most significant enhancement
Marzanna Strupińska et al.
Acta poloniae pharmaceutica, 66(2), 155-159 (2009-09-02)
Previously obtained picolinic acid benzylamide is a potent anticonvulsant with low neurotoxicity. In search for new effective anticonvulsants twelve new benzylamides (1-12) were synthesized and preliminary evaluated in the Anticonvulsant Screening Program (ASP) of Antiepileptic Drug Development Program (ADDP) of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service