304506
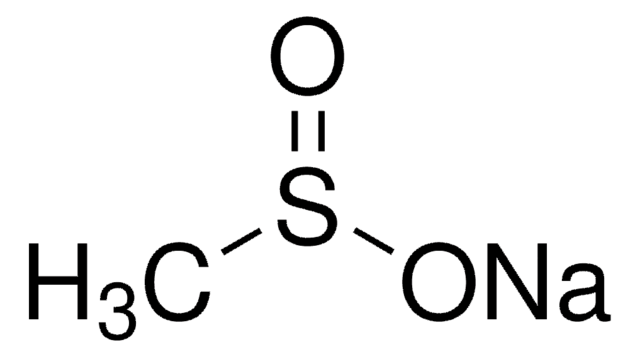
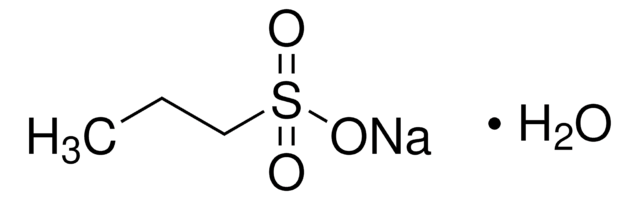
Sodium methanesulfonate
98%
Synonym(s):
Methanesulfonic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3SO3Na
CAS Number:
Molecular Weight:
118.09
Beilstein:
3915209
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
SMILES string
[Na+].CS([O-])(=O)=O
InChI
1S/CH4O3S.Na/c1-5(2,3)4;/h1H3,(H,2,3,4);/q;+1/p-1
InChI key
KKVTYAVXTDIPAP-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Sodium methanesulfonate is generally used as an anion source [CH3SO3]− for synthesizing ionic liquids (PILs).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Novel imidazolium-based poly (ionic liquid) s with different counterions for self-healing.
Cui J, et al.
Journal of Material Chemistry A, 5(48), 25220-25229 (2017)
Nitrile-functionalized azepanium ionic liquids: Synthesis characterization and thermophysical properties.
Lethesh K C, et al.
Journal of Molecular Liquids, 221, 1140-1144 (2016)
Sunghoon Kim et al.
Nature communications, 10(1), 3703-3703 (2019-08-20)
A family of plant nuclear ion channels, including DMI1 (Does not Make Infections 1) and its homologs CASTOR and POLLUX, are required for the establishment of legume-microbe symbioses by generating nuclear and perinuclear Ca2+ spiking. Here we show that CASTOR
Laura Mortara et al.
Langmuir : the ACS journal of surfaces and colloids, 34(37), 11049-11057 (2018-08-22)
Zwitterionic micelles adsorb anions and several techniques were used to determine the specificity of this interaction. Although at a lower intensity, this adsorption can be compared to those observed in cationic micelles, which showed that interfacial dehydration is a fundamental
Xiangke Chen et al.
The journal of physical chemistry. B, 114(47), 15546-15553 (2010-11-11)
The molecular organization at the aqueous dimethyl sulfoxide (DMSO) and methanesulfonic acid (MSA) surfaces was investigated using vibrational sum frequency generation (VSFG) spectroscopy and molecular dynamics (MD) simulation. The molecular orientation of surface DMSO and MSA is deduced based on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service