301272
2-Bromoacetamide
98%
Synonym(s):
α-Bromoacetamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
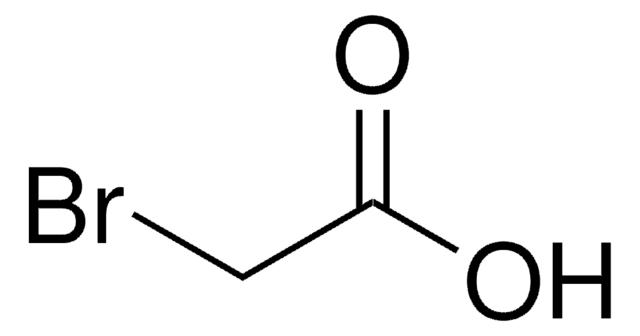
Linear Formula:
BrCH2CONH2
CAS Number:
Molecular Weight:
137.96
Beilstein:
1739073
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
87-91 °C (lit.)
functional group
amide
bromo
SMILES string
NC(=O)CBr
InChI
1S/C2H4BrNO/c3-1-2(4)5/h1H2,(H2,4,5)
InChI key
JUIKUQOUMZUFQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Kinetics of reaction of 2-bromoacetamide with a 39 base pair duplex DNA sequence was studied.
Application
2-Bromoacetamide was used in preparation of (2-carbamoylmethoxy-5-chloro-benzyl)-carbamic acid tert-butyl ester. It was aslo used as precursor to dehydropeptidase I inactivator.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shunke Ding et al.
Water research, 143, 325-333 (2018-07-10)
The effective removal of haloacetamides (HAMs) as a group of emerging disinfection by-products is essential for drinking water safety. This study investigated the degradation of 10 HAMs, including chlorinated, brominated, and iodinated analogues, by sodium sulfite (S(IV)) and the mechanism
Targeting renal dipeptidase (dehydropeptidase I) for inactivation by mechanism-based inactivators.
Y Q Wu et al.
Journal of medicinal chemistry, 34(6), 1914-1916 (1991-06-01)
M J Taylor et al.
Bioconjugate chemistry, 8(3), 354-364 (1997-05-01)
Attachment of a nondiffusible bromoacetyl electrophile to the 5-position of a thymine at the 5'-end of a pyrimidine oligodeoxyribonucleotide affords sequence-specific alkylation of a guanine base in duplex DNA two base pairs to the 5'-side of a local triple-helical complex.
Ni Cheng et al.
Journal of colloid and interface science, 511, 215-221 (2017-10-14)
Studies on supramolecular gel aggregates via supramolecular interactions are very fascinating and impressive. The interactions of gelator with solvent and inorganic salt can functionalize, activate, or control the properties of supramolecular gels. A lot of work on this area has
Yunkun Qian et al.
Water research, 186, 116346-116346 (2020-09-01)
Haloacetonitriles (HANs) and haloacetamides (HAMs) are nitrogenous disinfection byproducts that are present in filter backwash water (FBW) and sedimentation sludge water (SSW). In many cases FBW and SSW are recycled to the head of drinking water treatment plants. HAN and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service